- EN - English
- CN - 中文
Studying Cargo Transport Using RudLOV
利用 RudLOV 研究细胞内物质运输
发布: 2025年10月20日第15卷第20期 DOI: 10.21769/BioProtoc.5468 浏览次数: 1523
评审: Sébastien GillotinAnonymous reviewer(s)
Abstract
Most membrane and secreted proteins are transported from the endoplasmic reticulum (ER) to the Golgi apparatus and subsequently directed to their final destinations in the cell. However, the mechanisms underlying transport and cargo sorting remain unclear. Recent advancements in optical microscopy, combined with synchronized cargo protein release methods, have enabled the direct observation of cargo protein transport. We developed a new optically synchronized cargo release method called retention using the dark state of LOV2 (RudLOV). This innovative technique offers three exceptional control capabilities: spatial, temporal, and quantitative control of cargo release. RudLOV uses illumination to trigger transport and detect cargo. Consequently, the selection of an appropriate laser and filter set for controlling the illumination and/or detection is crucial. The protocol presented here provides step-by-step guidelines for obtaining high-resolution live imaging data using RudLOV, thereby enabling researchers to investigate intracellular cargo transport with unprecedented precision and control.
Key features
• RudLOV is a new optically synchronized intracellular cargo transport method.
• RudLOV enables precise spatial, temporal, and quantitative control of cargo release.
• RudLOV allows cargo to be released using a 445 or 488 nm laser with less damage than UV.
• RudLOV allows you to hook and release cargo without the use of exogenous chemicals.
Keywords: Live imaging (活细胞成像)Graphical overview
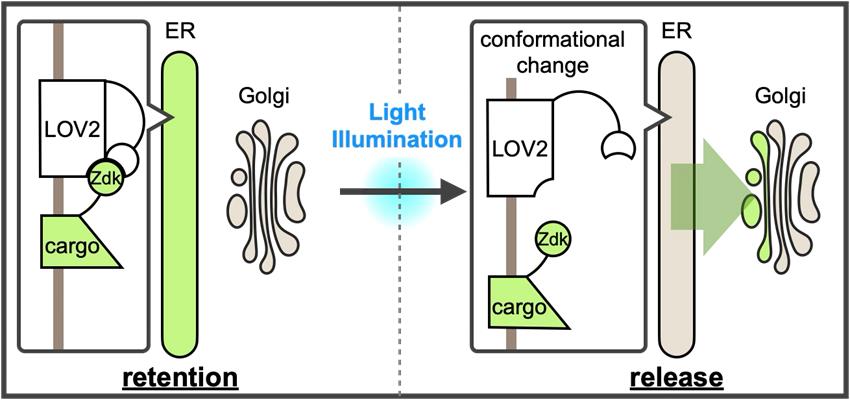
A new optically synchronized intracellular cargo transport method, RudLOV. In the dark, LOV2 binds to the Zdk1-fused cargo in the endoplasmic reticulum (ER). Light illumination triggers a conformational change in LOV2 that releases the cargo from the ER. RudLOV provides precise spatial and temporal control of cargo release. RudLOV functions independently of external chemicals.
Background
Approximately 30% of the cellular proteins are synthesized in the endoplasmic reticulum (ER) and transported to the cis-side of the Golgi apparatus. These proteins, referred to as cargoes, traverse the Golgi apparatus and arrive at the trans-Golgi network (TGN), a cargo-sorting station located on the trans-side of the Golgi apparatus [1,2]. In the TGN, the cargo is sorted and transported to its destination or secretion. However, the mechanisms underlying the intracellular cargo transport and sorting remain unclear. Recent advances in optical microscopy, combined with methods for the synchronized release of cargo proteins fused to fluorescent proteins or tags, have enabled the real-time imaging of their journey from sequestered organelles to their destinations [3,4]. Direct observation of cargo protein transport and sorting is crucial for elucidating the mechanisms underlying these processes.
Several methods have been developed to synchronize cargo trafficking. In mammalian cells, the retention using the selective hooks (RUSH) system developed by Boncompain et al. [5] is widely used. The RUSH system is based on two key components: cargo fused to a streptavidin-binding peptide (SBP) and streptavidin fused to an organelle retention signal as a hook. The interactions between these proteins retain cargo within the organelle. The addition of biotin triggers the synchronized release of cargo proteins because it has a higher affinity for streptavidin than that of SBP. Although the RUSH system is easy to use and useful for cells that are accessible to externally applied biotin, the administration of biotin is not feasible for some cells within organisms. Furthermore, the time from biotin administration to the initiation of cargo release varies between cells. Optical cargo release methods have also been reported. In one approach, multiple UVR8-fused cargo proteins are sequestered in the ER, and a brief pulse of UV light (~310 nm) triggers cargo exit from the ER [6]. The zapalog-mediated ER trap (zapERtrap) uses UV light (405 nm) to trigger the breakdown of zapalog, which is the ER trap mechanism, allowing cargo release from the ER [7]. Both systems enable the precise control of illumination at the single-cell or subcellular level using the same microscope employed for subsequent live imaging, providing exceptional spatial and temporal accuracy for cargo release. Although these optical cargo-releasing methods are advantageous, UV light can potentially damage cells, and zapERtrap requires a continuous supply of relatively expensive zapalogs.
We recently developed a new optically synchronized cargo release method called retention using the dark state of LOV2 (RudLOV) [8], which is based on LOVTRAP (LOV2 Trap and Release of Protein) [9]. LOVTRAP is an optogenetic system consisting of LOV2 and Zdk1 tags, capable of repeatedly and reversibly controlling protein binding with precise kinetics. LOV2 is the photosensor domain of Avena sativa phototropin with an absorption maximum of 450 nm that utilizes the endogenous cofactor flavin mononucleotide (FMN) as a chromophore. Zdk1, an engineered aptamer that selectively binds to the dark state of LOV2, was developed by mRNA display screening of a library based on the Z-domain of protein A. LOVTRAP uses LOV2 as a trap for Zdk1-tagged proteins, which can be reversibly released by blue light illumination. LOV2 in the light state is spontaneously restored to the dark state, with a half-life of approximately 9 s in the case of wild-type Avena sativa LOV2.
In RudLOV, the cargo is fused to Zdk1, which is captured in the dark by LOV2 fused to ER-localized Sec61β. Mild blue light illumination (445 or 488 nm) induces a conformational change in LOV2 (-10 ms [10]), releasing Zdk1-fused cargo, which then synchronously exits the ER. The release of cargo from the ER occurs simultaneously in all illuminated cells (Figure 1F, G in Tago et al. [8]). The LOV2 conformation spontaneously reverts to the dark form within 55–81 s and recaptures the cargo with Zdk1 [11]. This mechanism allows control of the amount of cargo released by adjusting the strength and duration of illumination. We successfully demonstrated the laser power- and duration-dependent release of cargo, as well as cargo release in a single cell and even in a single Golgi stack (Figure 2A, B in Tago et al. [8]).
Live imaging requires the detection of fluorescent proteins fused to the cargo. Because both the transport trigger and cargo detection use illumination, careful selection of laser and filter sets to control illumination and/or detection is crucial for the successful implementation of RudLOV. Here, we present a step-by-step protocol for using RudLOV.
Materials and reagents
The materials, reagents, and plasmids used have been previously described [8].
Biological materials
1. HeLa cells (H. sapiens) (ATCC_CCL-2)
Plasmids
The following plasmids were deposited at Addgene (Table 1):
1. CMV-hSec61β-TagBFP2-linker-LOV2, a plasmid vector expressing ER-hook (used only for hook/cargo-co-expression) (Figure 1a)
2. Cargo-expressing vectors such as CMV-SP-IPVNTT-Zdk1-GPI (used only for hook/cargo co-expression)
3. pEB-puro-hSec61β-TagBFP2-linker-LOV2, an EBV-based episomal vector for easy establishment of stable transformants via puromycin selection (used when establishing stable hook-expressing lines)
4. CT7-cSec61β-TagBFP2-linker-LOV2-IRES2-SP-IPVNTT-Zdk1-FluorescentProtein-cargo (all-in-one vector) (Figure 1b).
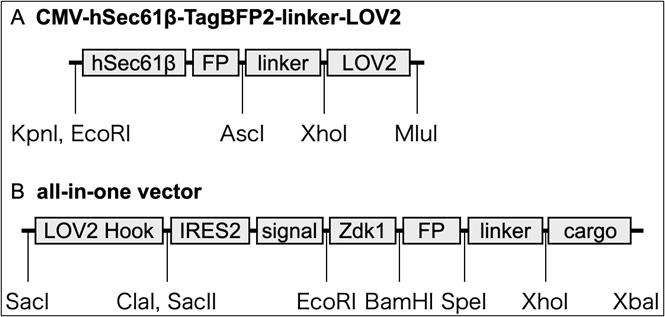
Figure 1. Model of RudLOV plasmids. (A) CMV-hSec61β-TagBFP2-linker-LOV2. (B) All-in-one vector. These plasmids show the major domains and unique cut sites. Abbreviations: FP, fluorescent protein (NeonGreen, Clover, Scarlet, and Halotag7); IRES2, internal ribosome entry site; signal, signal peptide (SP) and ER export signal (IPVNTT); cargo, cargo protein of interest. For more information, see Addgene.
Table 1. List of plasmids and Addgene ID#
| Addgene ID# | Plasmids | Addgene ID# | Plasmids |
|---|---|---|---|
| 246600 | CT7-LOVHook-Zdk-HT-GPI | 246601 | CT7-LOVHook-Zdk-NG-GPI |
| 246602 | CT7-LOVHook-Zdk-Sca-GPI | 246603 | CT7-LOVHook-Zdk-HT-VSVG |
| 246604 | CT7-LOVHook-Zdk-NG-VSVG | 246605 | CT7-LOVHook-Zdk-Sca-VSVG |
| 246606 | CMV-hSec61b-tBFP-LL-LOV2 | 246607 | CMV-Zdk-HT-GPI |
| 246608 | CMV-Zdk-NG-GPI | 246609 | CMV-Zdk-Sca-GPI |
| 246610 | CMV-Zdk-HT-VSVG | 246611 | CMV-Zdk-NG-VSVG |
| 246612 | CMV-Zdk-Sca-VSVG | 246613 | CMV-TNFa-Zdk-HT |
| 246614 | CMV-TNFa-Zdk-NG | 246615 | CMV-TNFa-Zdk-Sca |
The plasmid vectors for RudLOV were based on those of the RUSH system, with the Str-KDEL hook and SBP replaced with Sec61b-LOV2 and Zdk1, respectively. Similar to the RUSH system, there are separate plasmid vectors expressing hook or cargo, as well as all-in-one vectors expressing hook and cargo (see General notes 1–5).
Reagents
1. D-MEM (high glucose) (FUJIFILM Wako Chemicals, catalog number: 045-30285)
2. Penicillin-streptomycin-L-glutamine solution (×100) (FUJIFILM Wako Chemicals, catalog number: 161-23201)
3. 100 mmol/L sodium pyruvate (FUJIFILM Wako Chemicals, catalog number: 190-14881)
4. 1 mol/L HEPES buffer (Nacalai Tesque, catalog number: 17557-94)
5. Fetal bovine serum (Nichirei, catalog number: 174012)
6. D-PBS(-) (FUJIFILM Wako Chemicals, catalog number: 043-29791)
7. 0.25% Trypsin/EDTA (FUJIFILM Wako Chemicals, catalog number: 201-18841)
8. Nocodazole (Cayman Chemicals, catalog number: 13857)
9. Cycloheximide (Cayman Chemicals, catalog number: 14126)
10. Biotin (FUJIFILM Wako Chemicals, catalog number: 029-08713)
11. Biliverdine (TRC, catalog number: B386400)
12. JetPrime (Polyplus, catalog number: 101000015)
13. JetOptimus (Polyplus, catalog number: 101000006)
14. G418 sulfate (FUJIFILM Wako Chemicals, catalog number: 078-05961)
15. Puromycin dihydrochloride (FUJIFILM Wako Chemicals, catalog number: 160-23151)
Solutions
1. Cell culture medium (see Recipes)
Recipes
1. Cell culture medium
| Reagents | Final concentration | Volume |
|---|---|---|
| D-MEM (high glucose) | 91% | 500 mL |
| Fetal bovine serum | 5% | 25 mL |
| Penicillin-streptomycin-L-glutamine solution (×100) | 1% | 5 mL |
| 100 mmol/L sodium pyruvate | 1% | 5 mL |
| 1 mol/L HEPES buffer | 2% | 10 mL |
| Total | 100% | 545 mL |
Equipment
1. Inverted confocal laser scanning microscope with appropriate configuration. It is better to utilize a 514 nm laser in addition to 488 nm because 488 nm considerably activates LOV2 (see General note 9). A laser of 445 nm or similar wavelength is recommended for LOV2 conversion, although a white LED can also be used (see General note 7). We successfully performed RudLOV using the following instruments:
a. Confocal laser scanning microscope with 405/445/514/561/640 nm laser (Evident, model: FV3000). For observations without DM-switching, we strongly recommend customization with a multi-band dichroic beam splitter compatible with 445 and 514 nm lasers, such as the 405/445/514/561/640 pentaband beam splitter (Chroma 89903bs).
b. Confocal laser scanning microscope with 485–790 nm white laser + 445 nm laser (Leica, model: STELLARIS 5). This instrument has flexibility in both the excitation and detection wavelengths.
2. Stage-top incubators (STXF-WSKMX-SET and STXG-IX3WX-SET)
3. Standard cell culture equipment
4. Glass bottom dish or chamber slides with coverslip bottom, such as µ-slide 8 well (ibidi, catalog number: 80826)
5. Sharp long-pass filter to block light shorter than 520 nm from transmitted illuminator, such as SC 52, 7.5 × 7.5 cm (FUJIFILM)
Software and datasets
1. Olympus cellSens (https://evidentscientific.com/ja/products/software/cellsens)
2. Olympus FV31S-SW FLUOVIEW (https://evidentscientific.com/en/downloads)
3. Leica LAS-X (https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/?country=US)
4. ImageJ/Fiji [12]
5. Volocity 6.5.1 (https://www.volocity4d.com/)
Procedure
文章信息
稿件历史记录
提交日期: Mar 18, 2025
接收日期: Sep 9, 2025
在线发布日期: Sep 22, 2025
出版日期: Oct 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Tago, T., Satoh, T. and Satoh, A. K. (2025). Studying Cargo Transport Using RudLOV. Bio-protocol 15(20): e5468. DOI: 10.21769/BioProtoc.5468.
分类
细胞生物学 > 基于细胞的分析方法 > 细胞器能动性
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












