- EN - English
- CN - 中文
Generation of Agarose-Based FFPE Cancer Organoids for Morphology Preservation
制备基于琼脂糖的 FFPE 癌类器官以保持形态学特征
(*contributed equally to this work, § Technical contact) 发布: 2025年10月05日第15卷第19期 DOI: 10.21769/BioProtoc.5467 浏览次数: 1702
评审: Sérgio RibeiroAnonymous reviewer(s)

相关实验方案

采用 Davidson 固定液和黑色素漂白法优化小鼠眼组织切片的免疫组化染色
Anne Nathalie Longakit [...] Catherine D. Van Raamsdonk
2025年11月20日 1565 阅读
Abstract
Formalin-fixed paraffin-embedded (FFPE) slides are essential for histological and immunohistochemical analyses of organoids. Conventional preparation of FFPE slides from organoids embedded in basement membrane extract (BME) presents several challenges. During the fixation step, dehydration often causes collapse of the BME, which normally supports the three-dimensional architecture of organoids. As a result, organoids may lose their original morphology, particularly in the case of cystic or structurally delicate types, leading to distortion and reduced reliability in downstream histological evaluation. Here, we introduce a straightforward protocol that improves the reliability of FFPE slide preparation for BME-based organoids by enhancing sample integrity and sectioning quality. By using 2% agarose as a mold during the embedding process, organoids grown in BME were effectively stabilized, enabling reliable preservation of their morphology throughout FFPE slide preparation. This method effectively addresses the difficulties in processing structurally delicate organoids and allows robust preparation of diverse cancer organoid morphologies—such as cystic, dense, and grape-like structures—while maintaining their native three-dimensional architecture. Our approach simplified the technical process while ensuring reliable histopathological analysis, making it a valuable tool for cancer research and personalized medicine.
Key features
• FFPE preparation applicable to diverse cancer organoid morphologies, including cystic, dense, and grape-like, while preserving three-dimensional architecture.
• Agarose molding allows intact retrieval and fixation of BME domes, preventing collapse and maintaining organoid 3D architecture during FFPE preparation.
• Compatible with diagnostic IHC/IF markers (pan-CK, CK19, p63, Ki-67, p53) across cancer organoids.
Keywords: FFPE (FFPE)Graphical overview
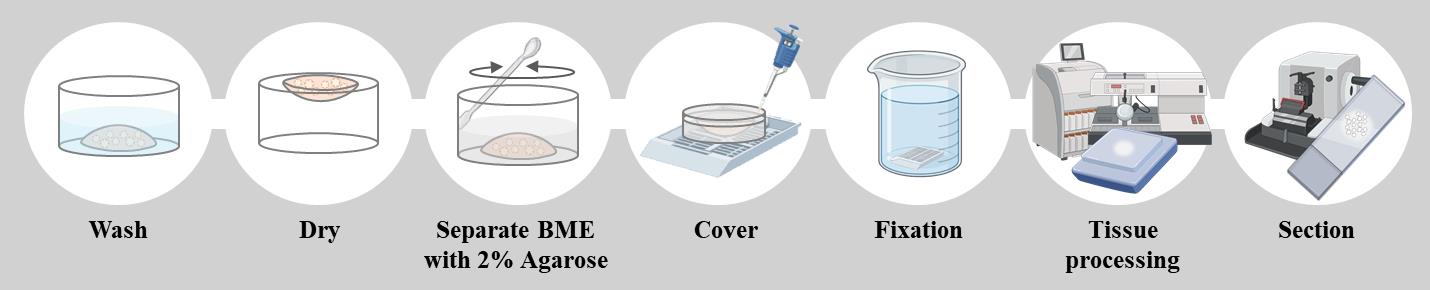
Schematic overview of the formalin-fixed paraffin-embedded (FFPE) organoid preparation protocol. Organoids cultured in BME are washed and partially dried before being embedded in 2% agarose. The agarose block is then fixed in formalin, processed for paraffin embedding, and sectioned for histopathological analysis. This method preserves organoid morphology and enables reliable staining for downstream applications such as H&E and immunostaining.
Background
Patient-derived organoids (PDOs) are regarded as reliable preclinical models for cancer research and personalized medicine [1]. They retain not only the structural complexity of the original tumor but also their biological behavior, including their responses to various therapeutic agents [2]. By faithfully recapitulating tumor-specific characteristics, PDOs offer a robust platform for testing novel therapeutics and developing individualized treatment strategies [3].
Importantly, PDOs display marked heterogeneity, and their morphology often reflects the underlying genetic and transcriptional features of the parental tumor [4–9]. For example, accumulation of mutations is frequently associated with dense or invasive organoids that can form grape-like structures, whereas organoids with lower mutational burden may maintain cystic or balloon-like morphologies resembling normal-like epithelial architecture. TP53 mutations, commonly present in head and neck, colorectal, and breast cancers, are associated with disorganized, compact structures lacking clear glandular architecture [10–12]. KRAS mutations, prevalent in pancreatic and colorectal cancers, often result in dense, multilayered organoids without lumen formation [13,14]. PIK3CA mutations lead to irregular, enlarged organoids in breast cancer [12], and CDH1 loss produces loosely cohesive or scattered organoids devoid of epithelial polarity in gastric cancer [4]. These genotype–phenotype relationships highlight the importance of preserving morphological integrity during sample preparation, as morphology serves as both a phenotypic marker and a functional readout of tumor biology.
Under laminin-rich basement membrane extract (BME), PDOs form distinct three-dimensional structures through enhanced self-renewal and self-organization, reinforcing their role in translational cancer research [15]. BME plays a critical role in maintaining polarity, lumen formation, and cystic morphology by providing a laminin- and collagen-rich extracellular scaffold [16]. However, during conventional FFPE processing, fixation often disrupts the BME, causing collapse of this structural support and leading to distortion or loss of normal-like organoid morphologies [17]. While dense organoids may partly preserve their structure through strong cell–cell adhesion, cystic or balloon-like morphologies are especially vulnerable once the BME is compromised [18].
This protocol was specifically designed to overcome the collapse of BME and distortion of fragile organoid morphologies that commonly occur during the dehydration steps of conventional FFPE processing. By functioning as a stabilizing matrix, the agarose mold immobilizes organoids within the BME, thereby preserving their native three-dimensional architecture. As a result, organoids maintain their original morphology, enabling accurate histological evaluation across a broad spectrum of organoid types, including cystic, dense, and grape-like structures.
The major strength of this approach lies in its ability to reliably preserve both structural integrity and histological detail, which are essential for downstream applications such as H&E staining, immunohistochemistry, and immunofluorescence. Although the method involves steps that may appear harsh—such as drying and the addition of warm agarose—and FFPE processing can impose certain limitations, molecular analyses that are highly sensitive to fixation stress may be restricted. Nevertheless, this protocol effectively supports morphological assessment and protein-level studies. In our validation, we confirmed the expression of cancer diagnostic markers such as CK19, pan-CK, and P53 and further demonstrated that marker expression relevant to subtype classification could be evaluated using tissue proteomics–based analysis [9,19–21]. Overall, this protocol provides a practical and robust method for generating high-quality FFPE organoid slides, making it well-suited for cancer research, biomarker evaluation, and translational applications in personalized medicine.
Materials and reagents
Biological materials
1. Patient-derived organoids from the tumor tissue of patients with pancreatic cancer
2. Patient-derived organoids from the tumor tissue of patients with gall bladder cancer
3. Patient-derived organoids from the tumor tissue of patients with bile duct cancer
4. Patient-derived organoids from the tumor tissue of patients with oral cancer
Reagents
1. DPBS (Hyclone, catalog number: SH30028.02)
2. Agarose (GenDEPOT, catalog number: A0100-010)
3. Surgipath Paraplast (Paraffin) (Leica, catalog number: 39601006)
4. Sterilized non-ionized water
5. 10% neutral buffered formalin (GDCHEM, catalog number: 010-1406-1010)
6. Xylene (Daejung, catalog number: 8587-4400)
7. Ethanol (DAEJUNG, catalog number: 4119-4410)
Solutions
1. 2% agarose solution (see Recipes)
2. 70% ethanol (see Recipes)
Recipes
1. 2% agarose solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Agarose | 2% (w/v) | 0.4 g |
| Deionized sterile water (DW) | n/a | 20 mL |
| Total | n/a | 20 mL |
2. 70% ethanol
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ethanol | 70% (w/v) | 210 mL |
| Deionized sterile water (DW) | n/a | 90 mL |
| Total | n/a | 300 mL |
Laboratory supplies
1. Embedding cassettes: Histosette I (SIMPORT, catalog number: HIS-M490-3)
2. Spatula (DAIHAN medical, catalog number: SL.Spa7011)
3. 50 mL beaker (DURAN, catalog number: BK1010-DU)
4. 10 mL disposable tips (Falcon, catalog number: 357551)
5. 200P tip (Axygen, catalog number: T-200-Y)
6. 1000P tip (Axygen, catalog number: T-1000-B)
7. 2 mL microcentrifuge tubes (Axygen, catalog number: MCT-200-C)
8. Glass jar (Lklab Korea, catalog number: H06-660-151)
9. Slide box (LABRYDAY, catalog number: HIP-1021B)
Equipment
1. Tissue processor (Leica, HistoCore, model: PEARL)
2. Heated paraffin embedding station (Lecia, HistoCore, model: Arcadia H)
3. Microtome (Leica, HistoCore, model: BIOCUT R)
4. -20 °C freezer
5. 4 °C refrigerator
6. Microwave oven (various suppliers)
7. Cold plate (Leica, model: EG1150 C)
8. Microscope (Leica, model: DM500)
9. Tissue float bath (JISICO, model: J-NBT)
10. ThermoE cooling and heating block (Bioer Technology, ThermoE, model: CHB-A4-2420)
11. Pipette-aid (DRUMMOND, model: HDR-4-000-201)
12. 200P and 1000P pipettes (GILSON, model: MyPIPETMAN)
Procedure
文章信息
稿件历史记录
提交日期: Jul 10, 2025
接收日期: Sep 1, 2025
在线发布日期: Sep 16, 2025
出版日期: Oct 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Lee, M. R., Kang, S., Jeon, A. R., Choi, S. W., Kong, S. Y. and Kim, Y. H. (2025). Generation of Agarose-Based FFPE Cancer Organoids for Morphology Preservation. Bio-protocol 15(19): e5467. DOI: 10.21769/BioProtoc.5467.
分类
癌症生物学 > 通用技术 > 细胞生物学试验
细胞生物学 > 组织分析 > 组织形态学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










