- EN - English
- CN - 中文
Detection of Plant RNA–Protein Interactions Using GFP-tag for Immunoprecipitation
利用 GFP 标签免疫沉淀检测植物 RNA–蛋白相互作用
发布: 2025年10月05日第15卷第19期 DOI: 10.21769/BioProtoc.5466 浏览次数: 1670
评审: Noelia ForesiDevendra Pratap SinghHarsimranjit Sekhon

相关实验方案

从大肠杆菌中大规模纯化III型毒素-抗毒素核糖蛋白复合物及其成分,用于生物物理学研究
Parthasarathy Manikandan [...] Mahavir Singh
2023年07月05日 2174 阅读

液体乳状液辅助纯化RNA结合蛋白(LEAP-RBP)快速高效分离总RNA结合蛋白质组
JohnCarlo Kristofich and Christopher V. Nicchitta
2024年07月20日 2593 阅读

用于Poly(A)纯化mRNA中N6-甲基腺苷定量的改良型m6A-ELISA方法
Wei Yee Chan [...] Folkert J. Van Werven
2025年06月20日 3006 阅读
Abstract
The study of RNA metabolism involves understanding how RNA molecules interact with specific RNA-binding proteins (RBPs). In plants, these interactions have traditionally been investigated using a variety of in vivo and in vitro approaches, such as electrophoretic mobility shift assays or the analysis of knockout mutants. More recently, immunoprecipitation-based techniques have been developed. Most of the available protocols rely on crosslinking procedures, magnetic beads, and RNA-seq as the final endpoint analysis. Here, we present a protocol developed to identify specific RNA targets that directly interact with known plant RBPs using GFP-Trap® agarose (ChromoTek) for immunoprecipitation without the need for crosslinking or RNA-seq. Briefly, a GFP-tagged RNA-binding protein is expressed in plant tissue, protein extracts are incubated with the GFP-Trap® agarose matrix, and the resulting complexes are isolated. Co-purified RNAs, specifically mRNAs, are then analyzed by RT-PCR to detect bound transcripts. This protocol was first implemented for the study of RNA–protein interaction in Arabidopsis thaliana. This approach presents high potential for analysis in other plant species as well as several advantages, such as its high specificity and low cost. Even though GFP-Trap® magnetic agarose (ChromoTek) has been used in plant systems to detect RNA–protein interactions, the protocol presented here consists of an alternative that is straightforward to implement when both candidate RNAs and RNA-binding proteins are known, and it can be broadly applied to study RNA–protein interactions in other plant systems.
Key features
• Can be used to confirm predicted RNA–protein interactions.
• Suitable for validating RNA–protein interactions when candidate transcripts and RBPs are already known.
• Compatible with downstream analysis by RT-PCR; can be adapted to RNA-seq if high-throughput data is needed.
• Does not require crosslinking or specialized equipment beyond standard molecular biology tools for direct and strong RNA–protein interactions.
Keywords: Plant RNA binding proteins (植物 RNA 结合蛋白)Graphical overview
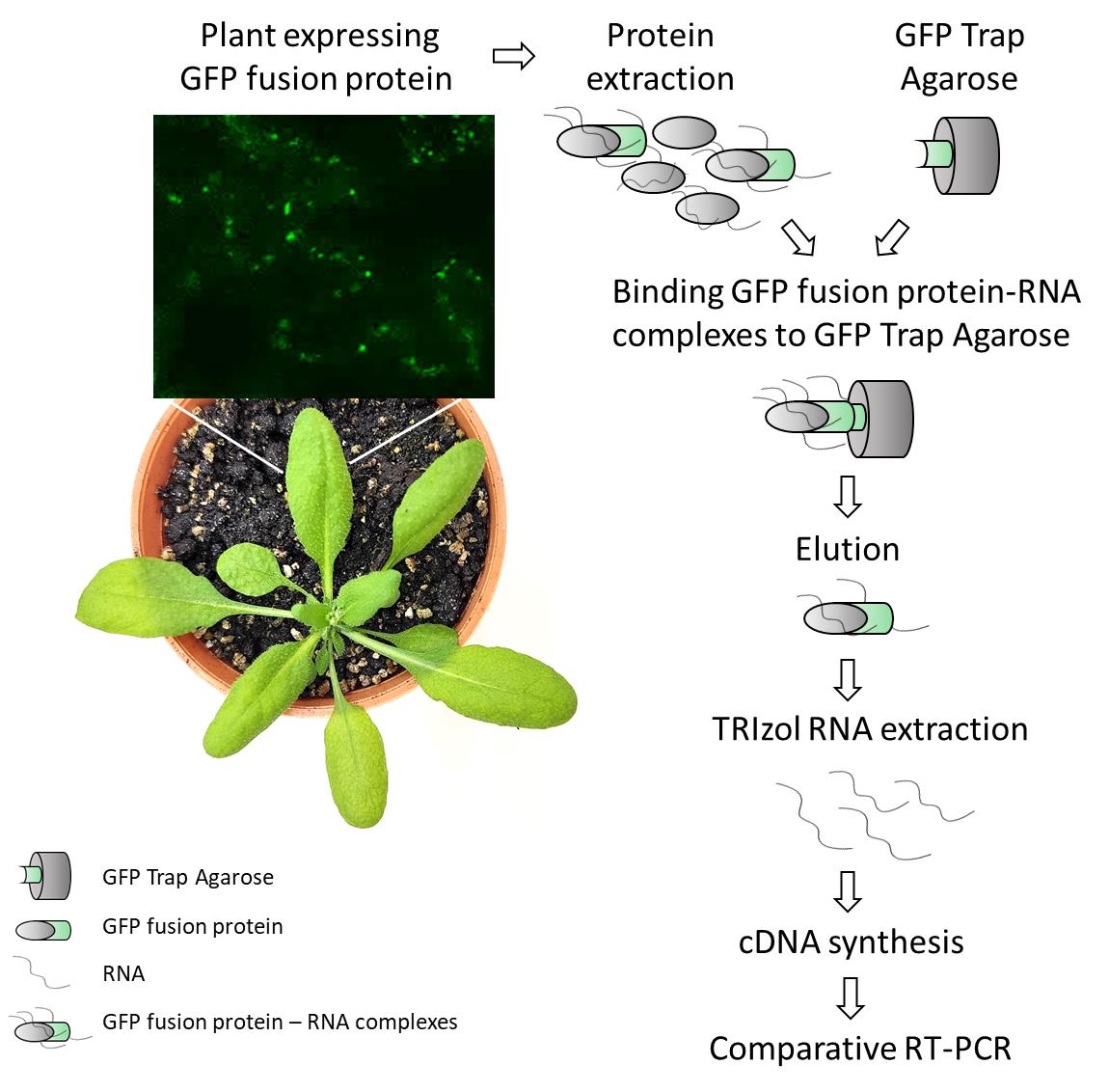
Detection of plant RNA–protein interactions using GFP-tag for immunoprecipitation from Arabidopsis thaliana leaf
Background
The field of protein–RNA interactions gained significant attention due to the complexity of RNA metabolism and the essential roles these interactions carry out to control RNA stability, localization, and translation. Different strategies have been employed to decipher such interactions, including a broad range of in vivo, in vitro, and in silico approaches [1,2]. In plants, initial attempts to demonstrate these interactions were carried out using methods such as nucleic acid-binding assays or mutant and knockout screening (cited in Burjoski et al. [3]). More recently, co-immunoprecipitation-based techniques have started to be developed. Even though these approaches have been developed primarily for mammalian systems, their application in plants is becoming increasingly common and useful (summarized in Burjoski et al. [3]; Mateos and Staiger [4]).
RNA immunoprecipitation (RIP) followed by RNA-seq (RIP-seq) is one of the most widely used techniques to study RNA–protein interactions in plants. A recent example is a report published in Bio-protocol, which demonstrated the identification of small RNA targets of GFP-tagged proteins by Small RNA-seq using GFP-Trap® magnetic agarose (ChromoTek) [5]. Here, we present a protocol for use when candidate transcripts and RNA-binding proteins (RBPs) are already known. Using the original protocol of GFP-Trap® agarose (developed for mammalian applications), we efficiently performed co-immunoprecipitation of RNA–protein complexes from Arabidopsis thaliana leaf. After elution, RNAs are extracted and reverse-transcribed for RT-PCR detection of specific mRNA targets. In addition to its high specificity and simplicity, the method described in this protocol offers other advantages, such as low cost, versatility, and quick steps, allowing a complete procedure within a few days, resulting in the identification of candidate RNA targets of plant-specific GFP-tagged RBPs. Although this protocol is primarily designed for the detection of known candidate RNAs, the in planta RBP co-purified RNA can also be subjected to RNA-seq for transcriptome-wide analysis. This makes it a valuable tool for targeted studies that can later be expanded to broader investigations, not only for leaf tissue but also for other plant organs.
Materials and reagents
Biological materials
1. Arabidopsis leaves from GFP-tagged protein of interest–expressing lines (protein of interest-GFP) and GFP-expressing lines (control line, see Note 2).
Notes:
1. In this protocol, leaves from 20-day-old Arabidopsis plants overexpressing GFP-tagged proteins were used. However, the protocol can be adapted for the analysis of RNA-protein interaction in seedlings and roots, as well as other plant species.
2. A control of a GFP protein is needed in order to discard nonspecific targets that can be bounded to GFP itself after co-IP. A useful control could be the overexpression of the GFP alone (for example, 35S::GFP, ideally with the same vector you selected for the overexpression of your protein of interest). An alternative would be overexpressing a GFP-tagged protein not belonging to the protein family of your protein of interest. In this protocol, ATG8a-GFP overexpressing lines were used as a negative control.
3. To be considered, the saturation of the GFP-Trap® agarose according to manufacturer recommendations corresponds to 12 µg of recombinant GFP protein per 10 µL of GFP-Trap® agarose.
Reagents
1. GFP-Trap® agarose (ChromoTek, catalog number: gta-20)
2. Tri (hydroxymethyl) aminomethane (Tris) (Genbiotech, catalog number: RU2510)
3. Ethylenediamine tetraacetic acid disodium salt (EDTA) (Biopack, catalog number: 1604.07)
4. Sodium chloride (NaCl) (J.T. Baker®, catalog number: 3624-19)
5. Sodium dodecyl sulfate (SDS) (Anedra, catalog number: 7211)
6. Triton X-100 (Biopack, catalog number: 2002.08)
7. Deoxycholate (Fluka, catalog number: 30970)
8. Magnesium chloride (MgCl2) (Promega, catalog number: A351H)
9. Phenylmethylsulphonyl fluoride (PMSF) (ICN Biochemicls Inc., catalog number: 195381)
10. Natrium azide (Na-Azide) (Merk, catalog number: S28222 917)
11. Glycine (Merck, catalog number: 4201)
12. Diethyl pyrocarbonate (DEPC) (Sigma, catalog number: 016K3726)
13. (Iso) propyl alcohol (anhydrous) (Mallinckrodt, catalog number: 3032-08)
14. Ethanol (Baker)
15. Chloroform (Biopack, catalog number: 1651.08)
16. Boric acid (Cicarelli, catalog number: 771214)
17. Agarose (Gene Biotech LE-Agarose 1200, catalog number: RU1010)
18. Ethidium bromide (Biobasic, catalog number: DB0195)
19. Hydrochloric acid (HCl) 36.5%–38% (Cicarelli, catalog number: 918110)
20. Liquid N2
21. M-MLV kit retrotranscriptase (Inbio, catalog number: K1601)
22. dNTPs set (Promega, catalog number: U1330)
23. Random primers (Macrogen Inc.)
24. Polymerase Taq-Pegasus kit (PB-L, catalog number: EA0102)
25. Specific primers designed to analyze putative targets (see Table 1)
Table 1. Primer list
| Primer name | Sequence | Citation | |
| atp8 | Fw | ACACTGACGACATGGTTCTACATTTAGCACTGGTGTATCCTATATGT | Takenaka et al. [6] |
| Rv | TACGGTAGCAGAGACTTGGTCTTGCTTCCTTGGCCATGTACA | Takenaka et al. [6] | |
| nad2int3ex4 | Fw | GGCGAATTTCAAACTTGTGG | Koprivova et al. [7] |
| Rv | TACGGTAGCAGAGACTTGGTCTTCCTTCTATGGTTCCACATGAGA | Takenaka et al. [6] | |
| nad2ex4 | Fw | ACACTGACGACATGGTTCTACACTTTCTATTGCGCCTAAAATCTCT | Takenaka et al. [6] |
| Rv | TACGGTAGCAGAGACTTGGTCTTCCTTCTATGGTTCCACATGAGA | Takenaka et al. [6] | |
26. 100 base pairs molecular marker (PB-L, catalog number: MA02)
27. TRIzol (Ambion by life technologies, catalog number: 15596018)
Solutions
1. Tris-HCl, pH 7.5, 1 M (see Recipes)
2. EDTA, pH 8, 0.5 M (see Recipes)
3. SDS 10% (see Recipes)
4. RIP A buffer (ChromoTek) (see Recipes)
5. Washing buffer (ChromoTek) (see Recipes)
6. Elution buffer (ChromoTek) (see Recipes)
7. Neutralization buffer (ChromoTek) (see Recipes)
8. DEPC water (see Recipes)
9. TBE buffer 5× (see Recipes)
Recipes
1. Tris-HCl, pH 7.5, 1 M
Weigh 12.11 g of Tris, add 75 mL of sterile water, adjust to pH 7.5 with HCl, and fill with sterile water to a final volume of 100 mL.
2. EDTA, pH 8, 0.5 M
Weigh 18.6 g of EDTA, add 75 mL of sterile water, adjust to pH 8 using 10 M NaOH, and stir until EDTA is completely dissolved. Solubility improves while alkalinity increases. Complete with sterile water to a 100 mL final volume.
3. SDS 10%
Dissolve 10 g of SDS in 100 mL of sterile water.
4. RIP A buffer (ChromoTek, adapted recipe)
10 mM Tris/Cl pH 7.5, 150 mM NaCl, 0.5 mM EDTA, 0.1% SDS, 1% TritonTM X-100, 1% deoxycholate, 0.09% Na-Azide, 2.5 mM MgCl2, 1 mM PMSF (adjust the pH to 7.5 at 4 °C).
5. Washing buffer (ChromoTek, adapted recipe)
10 mM Tris/Cl pH 7.5, 150 mM NaCl, 0.5 mM EDTA (adjust the pH to 7.5 at 4 °C), 0.018% Na-Azide, 1 mM PMSF.
6. Elution buffer
200 mM glycine pH 2.5 (adjust the pH at 4 °C).
7. Neutralization buffer
1 M Tris pH 10.4 (adjust the pH at 4 °C).
8. DEPC water
1 mL DEPC/L water; stir overnight and autoclave.
9. TBE buffer 5×
Dissolve 54 g of Tris and 27.5 g of boric acid in 500 mL of sterile water. Add 20 mL of EDTA pH 8, 0.5 M (Recipe 2) and complete the final volume up to 1 L of stock solution. For electrophoresis and gels, make a 10× dilution to obtain a 0.5× TBE buffer.
Notes:
1. Sterile water is obtained by autoclaving 40–70 µS/cm water. DEPC water is used for RNA extraction, cDNA synthesis, and RT-PCR steps.
2. We followed the manufacturer’s recommendations to adjust the pH of solutions at 4 °C. The procedure presented in this protocol is mainly performed at cold temperatures or on ice to prevent RNA or protein degradation, so the pH of solutions must be adjusted at 4 °C as the working temperature for the protocol.
Laboratory supplies
1. Mortar
2. Spatula
3. Centrifuge tubes 1.5 mL (Henso, conical bottom, sterile)
4. PCR tubes 0.2 mL (Henso, DNAse and RNAse free)
Equipment
1. Table centrifuge (Eppendorf, model: centrifuge 5418)
2. PCR machine (Applied BiosystemTM, Veriti 96 wells, 4375786)
3. Agarose gel electrophoresis equipment (Bio-Rad, model: PowerPacTM Basic Power Supply, Horizontal Electrophoresis Systems)
Procedure
文章信息
稿件历史记录
提交日期: Jul 11, 2025
接收日期: Sep 2, 2025
在线发布日期: Sep 16, 2025
出版日期: Oct 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Marchetti, F., Distéfano, A., Pagnussat, G. C. and Zabaleta, E. J. (2025). Detection of Plant RNA–Protein Interactions Using GFP-tag for Immunoprecipitation. Bio-protocol 15(19): e5466. DOI: 10.21769/BioProtoc.5466.
分类
植物科学 > 植物分子生物学
生物化学 > RNA > RNA-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link