- EN - English
- CN - 中文
Artificial Metalloenzymes in Artificial Sanctuaries Through Liquid–Liquid Phase Separation
利用液–液相分离构建人工金属酶反应微环境
发布: 2025年10月05日第15卷第19期 DOI: 10.21769/BioProtoc.5465 浏览次数: 1568
评审: Elena A. OstrakhovitchAnonymous reviewer(s)
Abstract
Artificial metalloenzymes (ArMs) hold great promise for expanding the toolbox of non-natural transformations usable in living systems, such as cells, plants, and animals. However, their practical application remains challenging, primarily due to their unsatisfactory stability and inefficient intracellular assembly. We recently reported a new strategy, called artificial metalloenzymes in artificial sanctuaries (ArMAS) through liquid–liquid phase separation (LLPS), to enhance the performance of ArMs in cells by placing them in more friendly artificial microenvironments. Here, this protocol describes the detailed method for using this ArMAS–LLPS strategy, a robust way to create artificial compartments using an ArM protein scaffold through LLPS and construct ArMs within using self-labeling cofactor anchoring reactions. In detail, in Escherichia coli, membraneless protein condensates are formed by expressing a self-labeling fusion protein, HaloTag-SNAPTag (HS) and act as intracellular sanctuaries. Simultaneously, the HS scaffolds enable site-specific, bioorthogonal conjugation with synthetic metal cofactors, facilitating efficient ArM formation within the LLPS domains. This strategy can significantly enhance the intracellular catalytic activity and stability of the named HS-based ArMs, allowing whole-cell catalysis to be performed to enable abiotic transformations both in vitro and in vivo. The protocol provides a proof-of-concept approach for researchers aiming to develop stable ArM-based whole-cell catalytic systems for synthetic biology and therapeutic applications.
Key features
• Describes a robust and reproducible protocol for constructing artificial metalloenzymes (ArMs) in living E. coli cells using protein-driven liquid–liquid phase separation (LLPS).
• Demonstrates how intracellular LLPS regions can serve as protective catalytic microenvironments, significantly improving ArM stability and catalytic turnover.
• Applicable to various abiotic catalytic transformations, including olefin metathesis.
Keywords: Artificial metalloenzyme (人工金属酶)Graphical overview
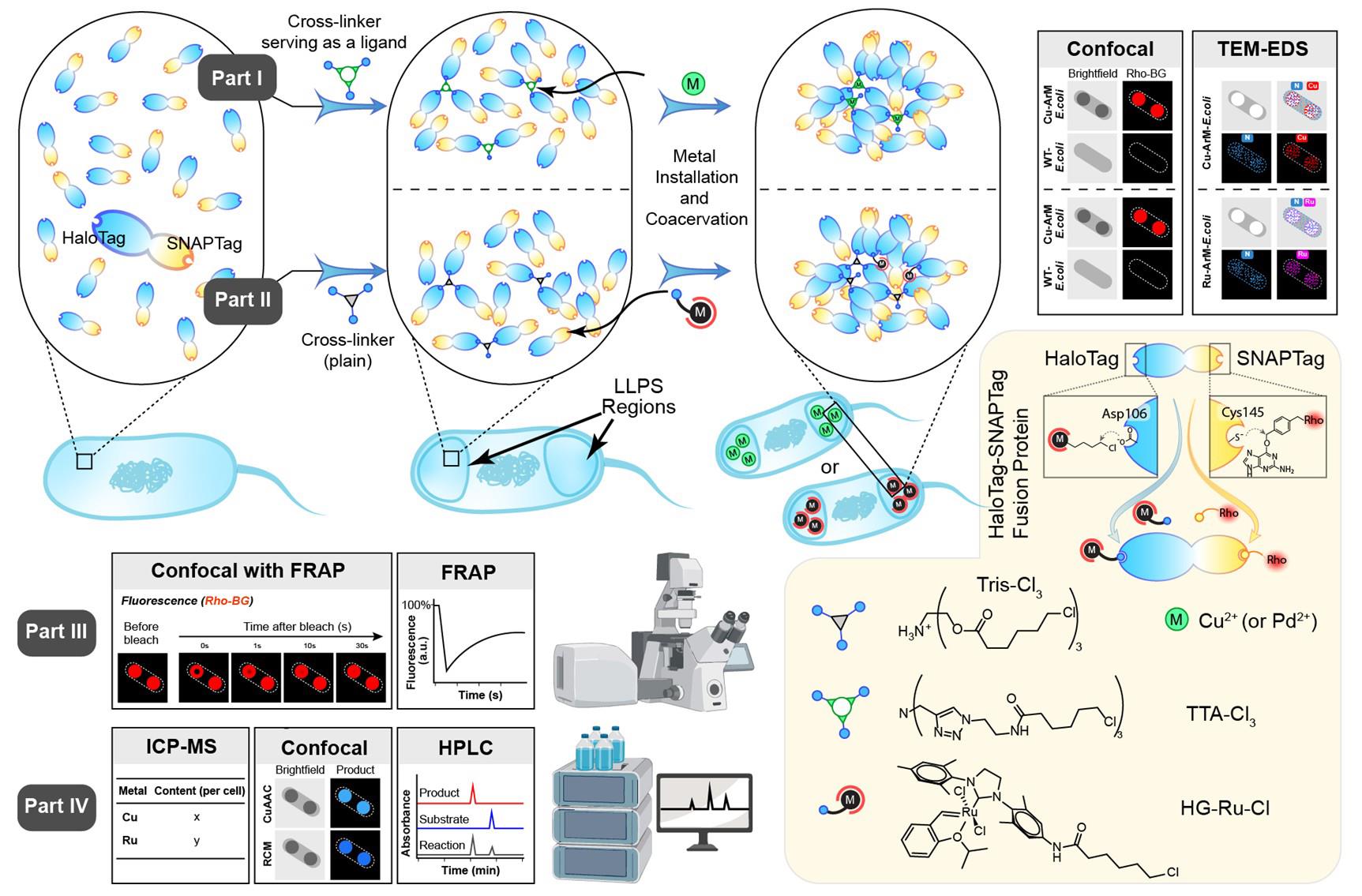
A cartoon illustration of the artificial metalloenzymes in artificial sanctuaries through liquid–liquid phase separation (ArMAS–LLPS) strategy using HaloTag-SNAPTag (HS) fusion protein as the artificial metalloenzyme (ArM) scaffold. In part I, a ligand-crosslinker was used to initiate the LLPS, followed by metal ion cofactor binding on the ligand to form ArMs inside the LLPS regions. In part II, a crosslinker without metal-coordinating function was used to initiate the LLPS, followed by metal complex cofactor anchoring on the HaloTag side of the HS scaffold to form ArMs inside the LLPS regions. In part III, fluorescence recovery after photobleaching (FRAP) analysis is performed to verify the liquid-like properties of the phase-separated protein. In part IV, various assays are conducted to evaluate the catalytic activity enhanced by the ArMAS–LLPS technology.
Background
Since ancient times, whole-cell catalysts have been widely used as natural tools for chemical transformation—from fermentation starters used in brewing to Penicillium species employed in penicillin production. These systems have played a critical role in enabling low-cost and large-scale manufacturing of chemicals. In recent years, whole-cell catalysts equipped with artificial metalloenzymes (ArMs) have emerged as a novel hybrid catalytic platform that has attracted growing attention [1,2]. ArMs integrate synthetic metal cofactors with natural protein scaffolds to catalyze abiotic reactions, thereby significantly expanding the scope of enzyme catalysis [4–9]. Incorporating ArMs into whole-cell systems not only enables a broad range of engineered reactions [10,11] but also helps to address challenges such as the high purification cost, limited environmental stability, and poor membrane permeability typically associated with ArMs delivery in cellulo and in vivo [1,12,13].
However, efficient integration of ArMs into whole cells remains challenging due to the complexity of anchoring synthetic metal cofactors onto protein scaffolds within the intracellular environment. Although strategies using high-affinity inhibitors or substrate analogs have been developed to simplify this process, they often fail to offer sufficient protection for sensitive metal cofactors, leading to limited ArM stability in vivo [14–16]. To address this issue, we proposed the artificial metalloenzymes in artificial sanctuaries (ArMAS)–liquid–liquid phase separation (LLPS) strategy, which aims at the construction of artificial intracellular compartments used as dedicated sanctuaries for abiotic catalysis, enhancing the intracellular stability of ArMs [17,18]. These compartments are formed via protein-mediated, minor crosslinking-initiated LLPS, which offers spatial segregation while allowing controlled exchange of materials with the surrounding cytoplasm.
This protocol describes the exact way to establish catalytic “shelters” by inducing controlled LLPS of ArM scaffold proteins, thereby protecting catalytic activity within the cell. HaloTag-SNAPTag (HS), a self-labeling fusion protein, is selected as the model ArM scaffold. The HaloTag side enables bioorthogonal covalent conjugation with alkyl chloride–modified metal cofactors so that ArMs can form, while the SNAPTag provides an orthogonal labeling handle for site-specific conjugation to benzylguanine-functionalized molecules. This dual-tag design not only allows efficient intracellular assembly of ArMs within the condensates formed by HS-LLPS but also offers a strategy to incorporate additional probes or modulators—such as fluorescent labels or crosslinkers—to track, stabilize, or tune the catalytic shelters. This protocol provides a detailed step-by-step procedure for each step in the creation of a whole-cell catalytic system based on ArMAS–LLPS in Escherichia coli, including protein expression, LLPS induction, ArM assembly in cellulo, and performance testing.
Materials and reagents
Biological materials
1. Competent cells E. coli strain BL21 (DE3) (Tsingke, catalog number: DLC201)
2. Plasmid containing HS protein: pET-21d-HS (Figure 1)
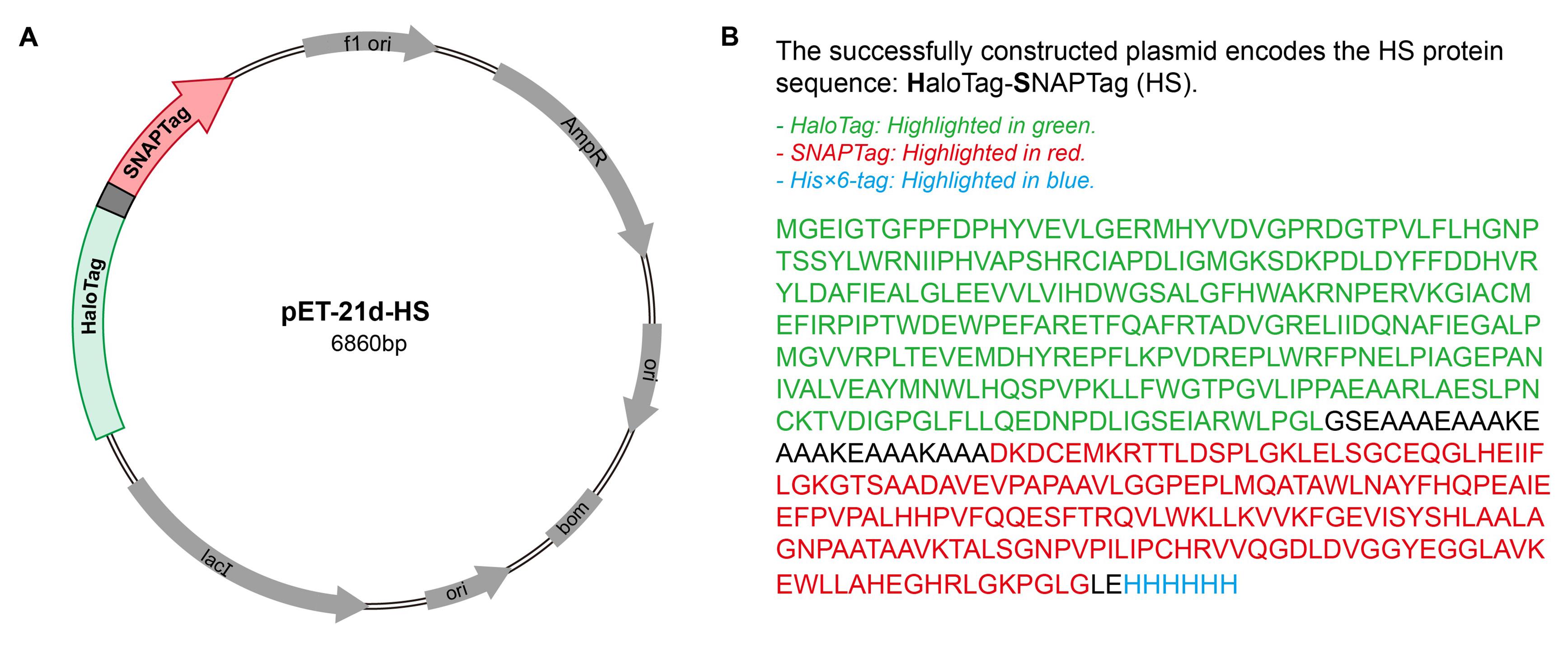
Figure 1. Plasmid map for the HaloTag-SNAPTag (HS) constructs used in the experiment. (A) Plasmid map of the HS expression construct in the pET-21d vector. (B) Amino acid sequence of the HS fusion protein.
Reagents
1. Ampicillin (Macklin, catalog number: A6265)
2. Dimethyl sulfoxide (DMSO) (Macklin, catalog number: D6258)
3. Methanol (MeOH) (Macklin, catalog number: M813907)
4. 4-Ethynylanisole (4-EA) (Macklin, catalog number: M813630)
5. 1,4-Dioxane (Macklin, catalog number: D807836)
6. Magnesium chloride (MgCl2) (Macklin, catalog number: M813763)
7. Sodium chloride (NaCl) (Macklin, catalog number: S805275)
8. Phosphate buffered saline (PBS), 1× (pH 7.4) (Servicebio, catalog number: G4202-500ML)
9. Acetonitrile (ACN) (Macklin, catalog number: A800362)
10. Glutathione (GSH) (Macklin, catalog number: G6268)
11. Copper(II) sulfate hydrate (CuSO4·5H2O) (Macklin, catalog number: 767480)
12. Sodium ascorbate (NaAsc) (Leyan, catalog number: LY-TRCS612200)
13. Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Bomeibio, catalog number: PI5502-25g)
14. LB medium mix powder (Hopebio, catalog number: HB0128)
15. Nitric acid 65% (SCR, catalog number: 10014518)
Solutions
1. LB medium (see Recipes)
2. IPTG 1 M (see Recipes)
3. CuSO4·5H2O 1 M (see Recipes)
4. NaAsc 1 M (see Recipes)
5. GSH 2 M (see Recipes)
6. TTA-Cl3 15.7 mg/mL (see Recipes)
7. Tris-Cl3 10.4 mg/mL (see Recipes)
8. Rhodamine-labeled benzylguanine (Rho-BG) 15.7 mg/mL (see Recipes)
9. 5-Hydroxy-2-vinylphenol acrylate (HVPA) 20 mM (see Recipes)
10. Diallylpropylene dibenzoate (DAPB) 100 mM (see Recipes)
11. Ethynyldiphenylmethyl allyl ether (EnDA) 200 mM (see Recipes)
12. 3-azido-7-hydroxycoumarin (AzHCou) 25 mM (see Recipes)
13. 4-EA 50 mM (see Recipes)
Note: The structures of TTA-Cl3, Tris-Cl3, Rho-BG, HVPA, DAPB, EnDA, and AzHCou are shown in Figure 2.
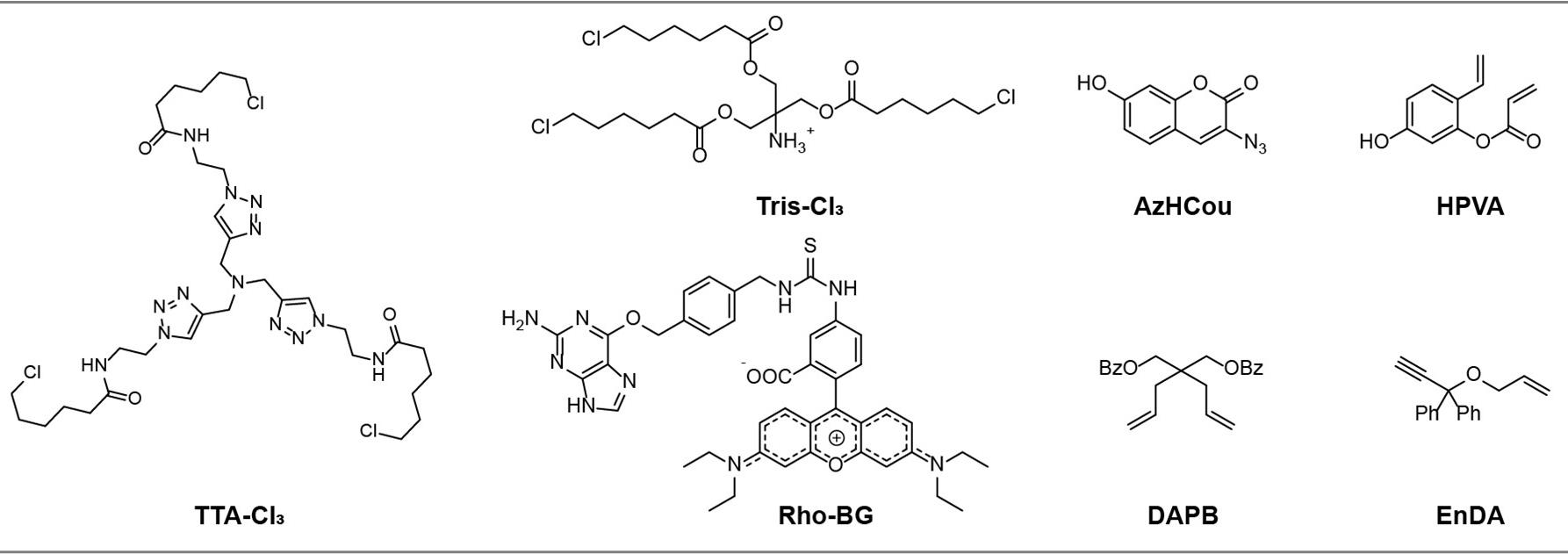
Figure 2. Chemical structures of non-commercial reagents used in this article. TTA-Cl3, Tris-Cl3, and Rho-BG were synthesized following [17]; AzHCou was synthesized following [19]; HVPA was synthesized following [20]; and DAPB and EnDA were synthesized following [21].
Recipes
1. LB medium (500 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| LB medium mix powder | 25 g/L | 12.5 g |
| ddH2O | n/a | 500 mL |
| Total | n/a | 500 mL |
Note: Autoclave at 121 °C for 30 min and aliquot. Store at RT.
2. IPTG 1 M (2 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| IPTG | 1 M | 476.6 mg |
| ddH2O | n/a | 2 mL |
| Total | n/a | 2 mL |
Note: Sterilize by filtration and store at -20 °C for up to one year.
3. CuSO4·5H2O 1 M (1 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CuSO4·5H2O | 1 M | 249.7 mg |
| ddH2O | n/a | 1 mL |
| Total | n/a | 1 mL |
4. NaAsc 1 M (1 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaAsc | 1 M | 198.1 mg |
| ddH2O | n/a | 1 mL |
| Total | n/a | 1 mL |
Note: Prepare the solution fresh each day and sterilize it by filtration before use.
5. GSH 2 M (2 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| GSH | 2 M | 1.229 g |
| ddH2O | n/a | 2 mL |
| Total | n/a | 2 mL |
Note: Sterilize by filtration and store at -20 °C for up to one year.
6. TTA-Cl3 15.7 mg/mL (1 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| TTA-Cl3 | 20 mM | 15.7 mg |
| DMSO | n/a | 1 mL |
| Total | n/a | 1 mL |
Note: Sterilize by filtration.
7. Tris-Cl3 10.4 mg/mL (1 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-Cl3 | 20 mM | 10.4 mg |
| DMSO | n/a | 1 mL |
| Total | n/a | 1 mL |
Note: Sterilize by filtration.
8. Rho-BG 15.7 mg/mL (1 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Rho-BG | 15.7 mg/mL (20 mM) | 15.7 mg |
| DMSO | n/a | 1 mL |
| Total | n/a | 1 mL |
Note: Sterilize by filtration.
9. HVPA 20 mM (1 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HVPA | 20 mM | 3.80 mg |
| DMSO | n/a | 1 mL |
| Total | n/a | 1 mL |
Note: Sterilize by filtration.
10. DAPB 100 mM (1 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HVPA | 100 mM | 36.44 mg |
| DMSO | n/a | 1 mL |
| Total | n/a | 1 mL |
Note: Sterilize by filtration.
11. EnDA 200 mM (1 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HVPA | 200 mM | 49.67 mg |
| DMSO | n/a | 1 mL |
| Total | n/a | 1 mL |
Note: Sterilize by filtration.
12. AzHCou 50 mM (0.5 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HVPA | 50 mM | 5.08 mg |
| DMSO | n/a | 0.5 mL |
| Total | n/a | 0.5 mL |
Note: Sterilize by filtration.
13. 4-EA 50 mM (0.5 mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HVPA | 50 mM | 3.30 mg |
| DMSO | n/a | 0.5 mL |
| Total | n/a | 0.5 mL |
Note: Sterilize by filtration.
Laboratory supplies
1. Automatic pipettes (ThermoFisher Scientific)
2. Pipette tips (Servicebio)
3. 2 mL microcentrifuge tube (Servicebio, catalog number: EP-200-M)
4. 15 mL centrifuge tube (Servicebio, catalog number: EP-1500-BJ)
5. 12 mL bacteria culture tube (Bikeman Bio, catalog number: 110408020)
6. Syringe filter (0.22 µm) (Merck Millipore, catalog number: C134954)
Equipment
1. Floor incubator shaker with cooling (Zhichu, model: ZQLY-180)
2. Biochemical incubator (Lichen, model: SPX-250B)
3. Ice machine (Meiling, model: MZB-25ZS)
4. Clean bench (Lichen, model: SW-CJ-1FD)
5. Biological safety cabinet (Esco, model: AC2-4S1)
6. Microplate reader (Thermo Fisher Scientific, model: Multiskan FC)
7. pH meter (Oustor, model: PHS-3C)
8. Fully automatic autoclave sterilizer (STIK, model: MJ-78A)
9. Vortex mixer (Scientific Industries, model: Vortex-Genie 2)
10. Centrifuge (Eppendorf, model: 5424R/5810R)
11. Microvolume spectrophotometer (Thermo Fisher Scientific, model: NanoDrop-2000)
12. Ultrapure water system (Merck Millipore, model: Elix 3)
13. Ultrasonic cell crusher (Scientz, model: JY92-IIN)
14. Confocal microscope (Nikon, model: A1RMP)
15. Confocal microscope with FRAP module (Carl Zeiss Microscopy, model: LSM980)
15. Inductively coupled plasma-mass spectrometer (ICP-MS) (Agilent Technologies, model: 7900)
16. High-performance liquid chromatography (HPLC) (Waters, model: 600; and Agilent Technologies, model: 1260)
17. Transmission electron microscope (TEM) equipped with an adapter for energy-dispersive X-ray spectroscopy (EDS) (JEOL, model: JEM-2100 Plus)
Procedure
文章信息
稿件历史记录
提交日期: Jun 22, 2025
接收日期: Sep 2, 2025
在线发布日期: Sep 16, 2025
出版日期: Oct 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Wang, K., Zhang, G., Zhang, L., Bai, Y. and Wu, T. (2025). Artificial Metalloenzymes in Artificial Sanctuaries Through Liquid–Liquid Phase Separation. Bio-protocol 15(19): e5465. DOI: 10.21769/BioProtoc.5465.
分类
生物工程 > 合成生物学
生物化学 > 蛋白质 > 合成
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












