- EN - English
- CN - 中文
Cost-Effective and Reproducible Preparation of mRNA-Loaded Lipid Nanoparticles Using a Conventional Laboratory-Scale Microfluidic Assembly System
利用常规实验室规模微流控组装系统制备高性价比且可重复的 mRNA 负载脂质纳米颗粒
发布: 2025年09月20日第15卷第18期 DOI: 10.21769/BioProtoc.5458 浏览次数: 1452
评审: David PaulNidhi MenonAnonymous reviewer(s)
Abstract
This protocol describes a standardized and economically accessible method for synthesizing mRNA-encapsulated lipid nanoparticles using routine laboratory equipment, including precision syringe pumps, Y-shaped glass microfluidic chips, and silicone tubing. Designed to address the cost and accessibility limitations of commercial microfluidic platforms, the system achieves performance metrics comparable to high-end devices while reducing equipment costs by 90%. By systematically optimizing hydrodynamic parameters (total flow rate: 12 mL/min; lipid-to-aqueous phase ratio: 3:1), the protocol enables consistent production of lipid nanoparticles with key quality attributes: high mRNA encapsulation efficiency (≥ 80%), narrow particle size distribution (100–120 nm, polydispersity index ≤ 0.2), and excellent storage performance (≥ 7 days at 4 °C ).
Key features
• A low-cost mRNA@LNPs synthesis method is developed using common lab equipment, cutting costs by 90% while matching commercial systems' performance.
• The LNP synthesis platform allows researchers to flexibly adjust hydrodynamic parameters to screen various lipid formulations.
Keywords: Laboratory preparation (实验室制备)Graphical overview
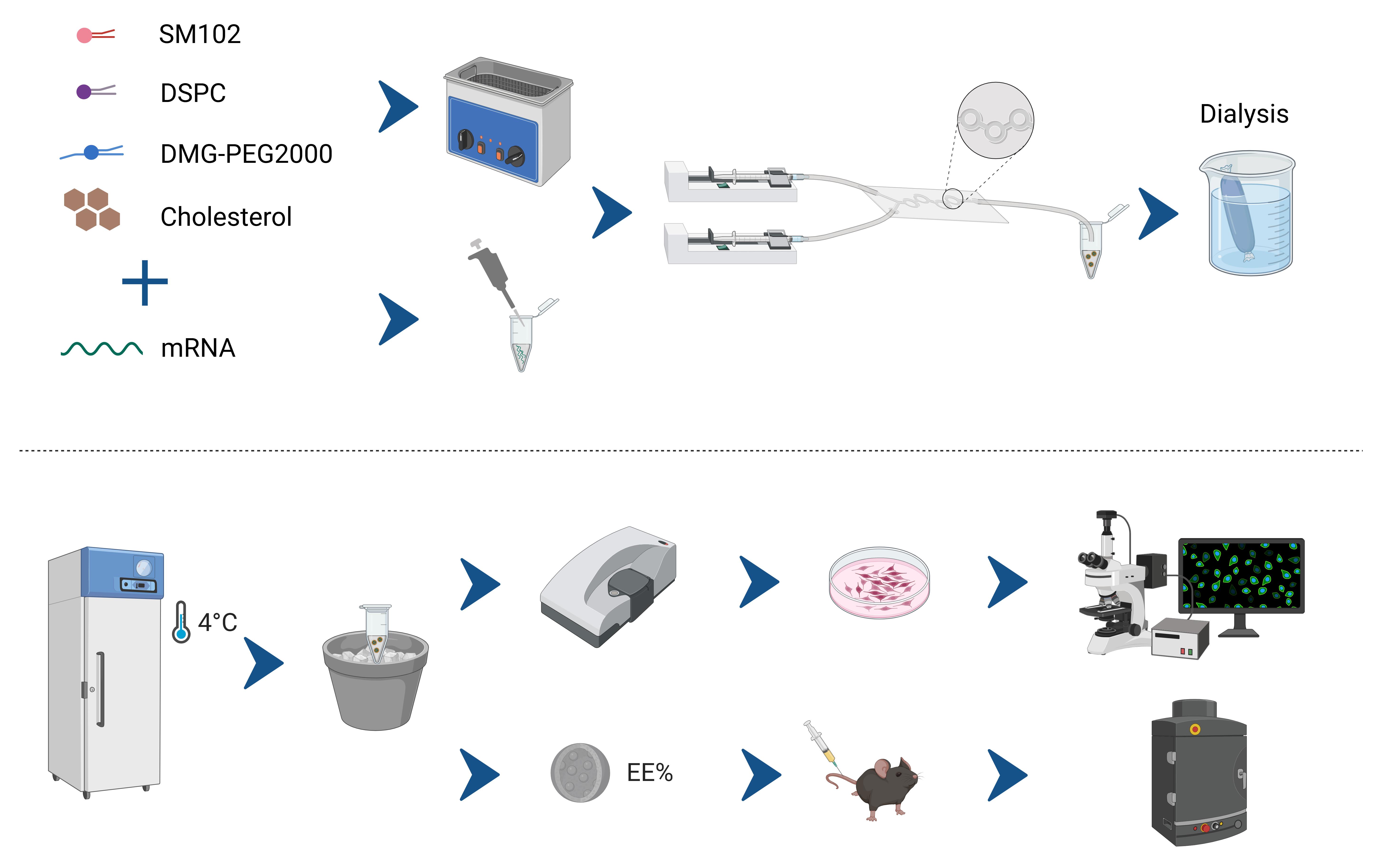
Schematic of the cost-effective microfluidic workflow for preparing mRNA-loaded lipid nanoparticles (mRNA@LNPs). The four lipids—SM102, DSPC, DMG-PEG2000, and cholesterol—are dissolved in anhydrous ethanol, while mRNA is dissolved in citrate buffer (4 °C). Both solutions are infused into a Y-junction glass microchip at a 3:1 (organic: aqueous) flow ratio. After on-chip self-assembly, the crude LNPs are dialyzed against PBS (4–6 h, 4 °C) and stored at 4 °C for up to one week. Downstream quality control includes RiboGreen-based encapsulation efficiency (EE%) determination and functional validation via in vitro and in vivo transfection assays.
Background
Lipid nanoparticles (LNPs) serve as core delivery vehicles for mRNA vaccines and gene therapy products. The scalability and economic viability of LNP production technologies govern both fundamental research and clinical applications [1]. While commercial microfluidic systems (e.g., FluidicLab LNP-S1, NanoAssemblr®) enable reproducible LNP synthesis through precision fluid control, their prohibitive costs (~$20,000–40,000 USD) and proprietary architectures pose significant constraints for academic laboratories [2]. Compared with the ~$35,000 USD price tag of commercial platforms such as the NanoAssemblr® Ignite, our complete system can be assembled for ~$4,000 USD—roughly one-tenth of the cost [3].
These limitations are most pronounced during multiparametric formulation screening and preclinical scaling, as evidenced during the COVID-19 pandemic, which exposed critical shortcomings in LNP technology for equitable vaccine development [4,5]. Current open-source alternatives (bulk mixing) reduce capital expenses but fail to achieve pharmaceutical standards, typically demonstrating compromised encapsulation efficiency (< 60%), broad size dispersity (PDI > 0.25), and inadequate batch-to-batch reproducibility [6,7].
To address these challenges, we developed a modular LNP synthesis platform using standard laboratory equipment that reengineers microfluidic principles through precision syringe pumps coupled with customized Y-junction glass chips. Compared with the commercial systems available on the market, our assembly method can also achieve efficient mRNA packaging by coordinating technical details and optimizing parameters. The encapsulation efficiency exceeds 80%, which is superior to the existing low-cost methods [8]. The prepared nanoparticles have a uniform particle size (80–120 nm, with a particle size distribution index of ≤ 0.2), and these particles have a long colloidal stability (they can remain stable for 7 days at 4 °C), which could meet WHO requirements for nucleic acid delivery systems [9,10]. This accessible platform facilitates the basic mRNA delivery system and vaccine research in laboratories with limited funds.
Materials and reagents
Biological materials
1. C57 mice, aged 8–10 weeks (SPF, Beijing Vital River Laboratory Animal Technology Co., Ltd.)
2. 293T cells (ATCC EY-X0525, China)
Reagents
1. SM102 (AVT, catalog number: O02010)
2. DSPC (AVT, catalog number: S01005)
3. Cholesterol (AVT, catalog number: O01001)
4. PEG2000-DMG (AVT, catalog number: O02005)
5. Anhydrous ethanol (Nanjing Chemical Reagent CO., Ltd, catalog number: C0691510123)
6. Firefly Luciferase (LUC) mRNA (N1-Me-Pseudo UTP, 1 mg/mL) (Vazyme, catalog number: DD4511)
7. eGFP mRNA (N1-Me-Pseudo UTP, 1 mg/mL) (Vazyme, catalog number: DD4503)
Note: If the laboratory prepares mRNA by itself, please refer to items 6 or 7 for quality control, which involves using the commercialized mRNA products from Vazyme, Jiangsu, China.
8. Citrate buffer (Absin, Shanghai, China, catalog number: abs9283)
9. 1× phosphate-buffered saline (PBS) (Servicebio, catalog number: G4250)
10. 1× DMEM basic (Thermo Fisher Scientific, catalog number: 6125052)
11. D-Luciferin potassium salt (Absin, catalog number: abs42075819)
12. Quant-iTTM RiboGreen® RNA Assay kit (Thermo Fisher Scientific, catalog number: R11490)
13. 20× TE buffer (Thermo Fisher Scientific, catalog number: T11493)
14. Triton X-100 (Beyotime, catalog number: ST797)
Solutions
1. 1× TE buffer (see Recipes)
2. 1% Triton X-100 buffer (see Recipes)
Recipes
1. 1× TE buffer
Dilute 20× TE buffer with reverse osmosis (RO) water (18.2 MΩ·cm, Millipore system, USA) to 1× concentration.
2. 1% Triton X-100 buffer
Dilute Triton X-100 with 1× TE buffer to a final concentration of 1%.
Laboratory supplies
1. Amicon® Ultra-15 centrifugal filters (MilliporeSigma, catalog number: UFC903096)
2. Slide-A-LyzerTM G2 dialysis cassettes (Thermo Fisher Scientific, catalog number: 66003)
3. 1.5 mL nuclease-free microcentrifuge tubes (Nest, catalog number: 615601)
4. 1 mL Luer-Lock syringes (Health Beauty, Shanghai, China)
5. 96-well cell culture plates (Nest, catalog number: 725031)
Caution: Ensure that all laboratory supplies are RNase-free to prevent RNA degradation.
Equipment
1. High-precision dual-syringe pump system (Wuhan Jianmi Zhikong Technology Co., Ltd, model: JSP0-02-1B)
2. Custom Y-junction glass microfluidic chip (Wuhan Jianmi Zhikong Technology Co., Ltd, model: JM LNP-B02-100/200, https://www.mifluidic.com/)
3. Chip fixture (https://www.taobao.com)
3. PTFE tubing assembly (Microfluidics & Lab-on-Chip Inc., Hubei, China)
4. Zetasizer Nano ZS (Malvern Panalytical, model: ZEN3600)
5. TEM system (Thermo Fisher Scientific, model: FEI Talos F200S G2)
6. AniView SE (Guangzhou Biolight Biotechnology Co., Ltd, Guangzhou, China)
7. Analytical balance (Jingqi Co., Ltd, model: FA2204T)
Procedure
文章信息
稿件历史记录
提交日期: Jun 17, 2025
接收日期: Aug 12, 2025
在线发布日期: Sep 3, 2025
出版日期: Sep 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Li, Y., Wu, M. and Zhao, R. (2025). Cost-Effective and Reproducible Preparation of mRNA-Loaded Lipid Nanoparticles Using a Conventional Laboratory-Scale Microfluidic Assembly System. Bio-protocol 15(18): e5458. DOI: 10.21769/BioProtoc.5458.
分类
分子生物学 > 纳米颗粒 > 植物源纳米颗粒
生物化学 > 脂质 > 脂质分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












