- EN - English
- CN - 中文
Rapid and Uniform NHS-Ester-Based Membrane Protein Labeling of Live Mammalian Cells
快速且均一的 NHS 酯基膜蛋白标记方法用于活体哺乳动物细胞
发布: 2025年10月05日第15卷第19期 DOI: 10.21769/BioProtoc.5455 浏览次数: 2189
评审: Rajesh RanjanAnonymous reviewer(s)
Abstract
Rapid and uniform labeling of plasma membrane proteins is essential for high-resolution imaging of dynamic membrane topologies and intercellular communication in live mammalian cells. Existing strategies for labeling live cell membranes, such as fluorescent fusion proteins, enzyme-mediated tags, metabolic bioorthogonal labeling, and lipophilic dyes, face trade-offs in the requirement of genetic manipulation, the presence of non-uniform labeling, the need for extensive preparation times, and limited choices of fluorophores. Here, we present a streamlined protocol that leverages N-hydroxysuccinimide (NHS)-ester chemistry to achieve rapid (≤5 min), covalent conjugation of synthetic small-molecule dyes to surface-exposed primary amines, enabling pan-membrane-protein labeling. This workflow covers dye stock preparation, labeling for suspension and adherent cells, multiplex live-cell imaging, fusion protein co-staining (including insulin-triggered receptor endocytosis), 3D membrane visualization, and in vivo assays for visualizing membrane-derived material transfers between donor and recipient cells using a lymphoma T-cell mouse model. This high-density labeling approach is compatible with various cell types across diverse imaging platforms. Its speed, versatility, and stability make it a broadly applicable tool for studying plasma membrane dynamics and intercellular membrane trafficking.
Key features
• Rapid high-density membrane labeling with small-molecule fluorescent dyes.
• Enables live-cell multiplexed imaging, amenable to primary cells and cells expressing fluorescent fusion proteins, and supports in vivo studies of membrane-associated cell–cell communications.
• Compatible with various fluorescence imaging modalities.
Keywords: NHS-ester labeling (NHS 酯标记)Graphical overview
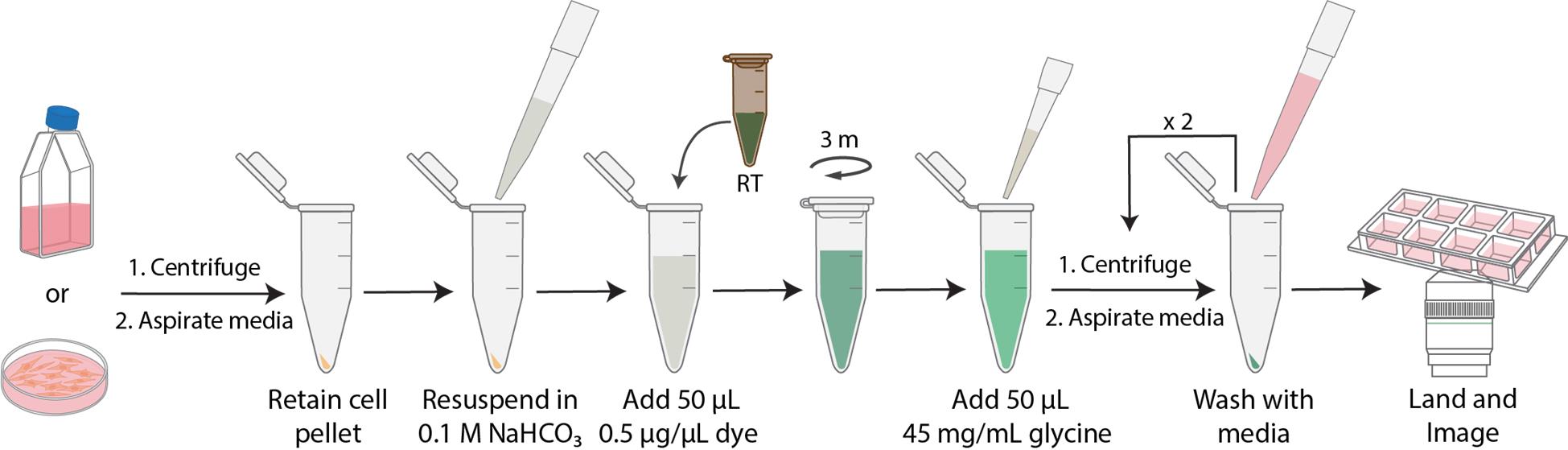
Workflow of N-hydroxysuccinimide (NHS)-ester dye labeling
Background
The plasma membrane hosts dynamic assemblies of proteins whose 3D topology and mobility underlie essential inter- and intracellular signaling. Direct visualization of complex 3D membrane topologies and dynamic protein distributions has greatly enhanced our understanding of how cells exchange information. Receptor-mediated signaling, specialized membrane protrusions (e.g., tunneling nanotubes), and extracellular vesicle release all depend on membrane protein organization [1,2]. Such dynamic platforms are critical for processes like immune synapse formation, epithelial barrier signaling, and neuronal communication. In fact, tunneling nanotubes (TNTs) have been observed connecting cells, enabling direct transfer of proteins, organelles, and electrical signals between cells [1,2]. Imaging of the plasma membrane thus provides needed insights into signal transduction, vesicular trafficking, and cell–cell communication in a diverse set of biological systems.
Despite advances in fluorescence microscopy, membrane labeling techniques remain limited. Protein-specific tags (e.g., fluorescent fusion proteins, HaloTag, SNAP-tag) allow visualization of individual proteins but require exogenous gene expression or enzyme-mediated labeling, which can disrupt native protein function or cell physiology [3,4]. Similarly, metabolic bioorthogonal labeling schemes can be labor-intensive and slow, limiting their routine use in live-cell experiments [5,6]. Other pan-membrane approaches use lipophilic dyes (DiI, DiO, DiD), which label membranes but often stain heterogeneously, have low membrane retention, and can form dye aggregates. Further, glycan-binding lectins can highlight certain membrane domains, but their binding varies with cell type and metabolic state, such as wheat germ agglutinin, which loses sensitivity in some pathological conditions [7]. Commercial covalent membrane stains, such as MemBrite, have been introduced, but they offer limited fluorophores and use proprietary chemistry, limiting transparency and versatility [8].
N-hydroxysuccinimide (NHS)-ester chemistry provides a rapid, uniform, covalent membrane labeling alternative. NHS-esters react instantly with surface-exposed primary amines on proteins to form stable amide bonds. The reaction is fast, allowing for high-density cell surface labeling with minimal dye internalization [1]. Because NHS-esters label all exposed amines indiscriminately, the resulting fluorescence is pan-membrane. The covalent linkage also yields very stable labeling, enabling prolonged live-cell imaging.
Overall, NHS-ester-based membrane labeling combines the speed, density, and uniformity of covalent chemistry with the flexibility of any synthetic dye. It does not require genetic modification and is compatible with several imaging modalities, e.g., total internal reflection fluorescence (TIRF), confocal, and 3D-structured illumination microscopy (SIM). The current protocol is optimized for common mammalian cell lines (Jurkat E6.1 T cells, DC2.4 dendritic cells, U2OS, and HEK293T) and mouse primary splenocytes but can be adapted for most adherent or suspension cells. It is particularly well-suited to live-cell microscopy and multiplex experiments and can be combined easily with fluorescent fusion proteins and small molecule probes as needed. Altogether, this rapid pan-membrane-labeling strategy provides a broadly applicable tool to visualize plasma membrane topology and dynamics in complex cellular systems.
Materials and reagents
Biological materials
1. DC 2.4 (MilliporeSigma, catalog number: SCC142)
2. HEK293T (ATCC, catalog number: CRL-3216)
3. Jurkat E6.1 (MilliporeSigma, catalog number: 88042803-1VL)
4. U2OS (ATCC, catalog number: HTB-96)
5. SNF5fl/fl, CD4-Cre+/- C57/BL6 mice (Jackson Laboratory)
6. Wildtype C57/BL6 mice (Jackson Laboratory)
Reagents
All reagents should be used within their manufacturer-recommended expiration or usage periods to ensure efficacy throughout experiments. Reagents designated as critical are necessary for the core labeling procedure, while non-critical reagents support additional labeling steps or optional protocol variations.
Critical:
1. Alexa FluorTM 647 NHS ester (Thermo Fisher Scientific, Invitrogen, catalog number: A37573)
2. Alexa FluorTM 488 NHS ester (Thermo Fisher Scientific, Invitrogen, catalog number: A20000)
3. NHS-Biotin (Lumiprobe, catalog number: 2551)
4. Sulfo-Cyanine3-NHS ester, 1 mg (Lumiprobe, catalog number: 11320)
5. Sulfo-Cyanine5-NHS ester, 1 mg (Lumiprobe, catalog number: 13320)
6. Alexa FluorTM 488 NHS ester, 1 mg (Lumiprobe, catalog number: 11820)
7. Sodium bicarbonate (NaHCO3), 500 g (MilliporeSigma, catalog number: S6014)
8. Glycine, 500 g (Fisher Scientific, catalog number: BP381-500)
Non-critical:
1. Roswell Park Memorial Institute (RPMI) 1640 cell culture media, 500 mL (Thermo Fisher Scientific, Gibco, catalog number: 11875093)
2. High-glucose Dulbecco’s modified Eagle medium (DMEM) cell culture media (4.5 g/L), 500 mL (Thermo Fisher Scientific, Gibco, catalog number: 11965118)
3. Low-glucose DMEM cell culture media (1 g/L), 500 mL (Thermo Fisher Scientific, Gibco, catalog number: 11885084)
4. Penicillin-streptomycin (Pen-Strep) 10,000 U/mL, 100 mL (Thermo Fisher Scientific, Gibco, catalog number: 1540-122)
5. Dulbecco’s phosphate-buffered saline (DPBS), 500 mL (Thermo Fisher Scientific, Gibco, catalog number: 14190-144)
6. DynabeadsTM, Human T-Activator CD3/CD28 (Thermo Fisher Scientific, Gibco, catalog number: 11161D)
7. Trypan blue solution, 0.4% (Thermo Fisher Scientific, Gibco, catalog number: 15250-061)
8. LipofectamineTM 2000, 1.5 mL (Thermo Fisher Scientific, Invitrogen, catalog number: 11668019)
9. Opti-MEM reduced serum media, 500 mL (Thermo Fisher Scientific, Gibco, catalog number: 51985034)
10. HEPES, 1 M, 100 mL (Fisher Scientific, Gibco, catalog number: 15630080)
11. Non-essential amino acids (NEAA) 100× solution (Fisher Scientific, Cytiva HyClone, catalog number: SH3023801)
12. Sodium pyruvate, 100 mM, 100 mL (Fisher Scientific, Gibco, catalog number: 11360070)
13. Bovine serum albumin (BSA), 100 g (Fisher Scientific, catalog number: BP9703100)
14. Acetonitrile, 8 mL (MilliporeSigma, catalog number: 900644)
15. Fetal bovine serum (FBS), 500 mL (MilliporeSigma, catalog number: F0926)
16. Dimethyl sulfoxide (DMSO), 500 mL (MilliporeSigma, catalog number: D8418-500mL)
17. Poly-L-lysine (PLL), 0.01%, 50 mL (MilliporeSigma, catalog number: P4707)
18. Chloroform, 1 L (MilliporeSigma, catalog number: 366919)
19. Methanol (MeOH), 1 L (MilliporeSigma, catalog number: 154903)
20. Recombinant human insulin, 100 mg (MilliporeSigma, catalog number: 11376497001)
21. Acetic acid, 100 mL (MilliporeSigma, catalog number: 695092)
22. Nucleofector 2b kit V (LONZA, catalog number: VCA-1003)
23. In vivo mAb anti-human CD3 (OKT3), 25 mg, lot: 809022M2 (BioXCell, catalog number: BE0001-2)
24. Paraformaldehyde (PFA), 16%, 100 mL (Electron Microscopy Sciences, catalog number: 15710)
25. FITC anti-mouse CD8α antibody, 0.1 mg (BD Biosciences, lot: 2248217, catalog number: 553030)
26. EasySep Mouse CD8+ T-cell Isolation kit (STEMCELL Technologies, catalog number: 19853)
27. EasySep Mouse CD4+ T-cell Isolation kit (STEMCELL Technologies, catalog number: 19852)
Solutions
1. DC 2.4 cell culture media (see Recipes)
2. HEK293T cell culture media (see Recipes)
3. U2OS cell culture media (see Recipes)
4. Jurkat E6.1 cell culture media (see Recipes)
5. Low-glucose DMEM cell culture media (see Recipes)
6. 0.1 M NaHCO3 (see Recipes)
7. 45 mg/mL glycine solution (see Recipes)
8. 200 nM recombinant human insulin + low-glucose DMEM (see Recipes)
Recipes
1. DC 2.4 cell culture media
| Reagent | Final concentration | Volume |
|---|---|---|
| RPMI 1640 media | n/a | 435 mL |
| FBS | 10% | 50 mL |
| Pen-Strep | 100 units/mL | 5 mL |
| HEPES (1 M) | 0.01 M | 5 mL |
| NEAA (100×) | 1× | 5 mL |
| Total | n/a | 500 mL |
Store at 4 °C for up to 3 months.
2. HEK293T cell culture media
| Reagent | Final concentration | Volume |
|---|---|---|
| High-glucose DMEM (4.5 g/L) | n/a | 440 mL |
| FBS | 10% | 50 mL |
| Pen-Strep | 100 units/mL | 5 mL |
| Sodium pyruvate (100 mM) | 1 mM | 5 mL |
| Total | n/a | 500 mL |
Store at 4 °C for up to 3 months.
3. U2OS cell culture media
| Reagent | Final concentration | Volume |
|---|---|---|
| High-glucose DMEM (4.5 g/L) | n/a | 440 mL |
| FBS | 10% | 50 mL |
| Pen-Strep | 100 units/mL | 5 mL |
| Sodium pyruvate (100 mM) | 1 mM | 5 mL |
| Total | n/a | 500 mL |
Store at 4 °C for up to 3 months.
4. Jurkat E6.1 cell culture media
| Reagent | Final concentration | Volume |
|---|---|---|
| RPMI 1640 media | n/a | 445 mL |
| FBS | 10% | 50 mL |
| Pen-Strep | 100 units/mL | 5 mL |
| Total | n/a | 500 mL |
Store at 4 °C for up to 3 months.
5. Low-glucose DMEM cell culture media
| Reagent | Final concentration | Volume |
|---|---|---|
| Low-glucose DMEM (1 g/L) | n/a | 440 mL |
| FBS | 10% | 50 mL |
| Pen-Strep | 100 units/mL | 5 mL |
| Sodium pyruvate (100 mM) | 1 mM | 5 mL |
| Total | n/a | 500 mL |
Store at 4 °C for up to 3 months.
6. 0.1 M NaHCO3
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaHCO3 | 0.1 M | 126 mg |
| H2O (ultra-pure water) | n/a | 15 mL |
| Total | n/a | 15 mL |
Filter with 0.22 μm. Store at 4 °C for up to 1 month.
7. 45 mg/mL glycine solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glycine | 45 mg/mL | 225 mg |
| H2O (ultra-pure water) | n/a | 5 mL |
| Total | n/a | 5 mL |
Filter with 0.22 μm. Store at 4 °C for up to 1 month.
8. 200 nM recombinant human insulin + low-glucose DMEM
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Recombinant human insulin (4 μM) | 200 nM | 10 μL |
| Low-glucose DMEM (1 g/L) | n/a | 190 μL |
| Total | n/a | 200 μL |
Recombinant human insulin is stored at -80 °C at 4 μM in PBS. Before each use, thaw and dilute insulin in low-glucose DMEM to 200 nM. Use the solution immediately.
Laboratory supplies
1. 8-well chambered cover glass, no. 1.5 (Cellvis, catalog number: C8-1.5-N)
2. 1.5 mL disposable microcentrifuge tubes (VWR, catalog number: 89000-028)
3. 0.5 mL amber screw-cap microtubes (Thermo Fisher Scientific, catalog numbers: 3422AS and 3471AS)
4. 0.22 μm syringe filter, PVDF, 25 mm (VWR, CellPro, catalog number: 104028-492)
5. Syringes, 10–12 cc, luer lock (Fisher Scientific, Exel International, catalog number: 14-841-54)
6. P10, P200, and P1000 pipettes and respective tips
Equipment
1. Biosafety cabinet (Thermo Fisher Scientific, model: 1300 series A2)
2. Benchtop microcentrifuge (Eppendorf, model: 5424)
3. Tube revolver rotator (Thermo Fisher Scientific, catalog number: 88881001)
4. 6-tube magnetic separation rack (New England Biolabs, catalog number: S1506S)
5. Acid Resistant Refrigerated CentriVap® Centrifugal Vacuum Concentrator (Labconco, catalog number: 7310022)
Software and datasets
1. NIS-Elements Advanced Research software (Nikon, version: 5.42.06)
2. Fiji (National Institutes of Health, version: 1.54 f)
Note: This protocol is adaptable to other image analysis and microscope control software.
Procedure
文章信息
稿件历史记录
提交日期: Jul 12, 2025
接收日期: Aug 25, 2025
在线发布日期: Sep 3, 2025
出版日期: Oct 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Burgess, A., Gunasekara, H. and Hu, Y. S. (2025). Rapid and Uniform NHS-Ester-Based Membrane Protein Labeling of Live Mammalian Cells. Bio-protocol 15(19): e5455. DOI: 10.21769/BioProtoc.5455.
分类
细胞生物学 > 细胞成像 > 活细胞成像
免疫学 > 免疫细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













