- EN - English
- CN - 中文
A Model of Breast Cancer Micrometastasis in a Three-Dimensional (3D) Liver Spheroid for Testing an Antimetastatic Therapy
用于抗转移治疗研究的三维肝脏类球乳腺癌微转移模型
发布: 2025年09月20日第15卷第18期 DOI: 10.21769/BioProtoc.5454 浏览次数: 3176
评审: Dipak Kumar PoriaKrishna Priya SyamaAnonymous reviewer(s)

相关实验方案

配体与HiBiT标记的GPCR在活细胞表面上的平衡和动力学测量
Michelle E. Boursier [...] Rachel Friedman Ohana
2020年12月20日 4794 阅读
Abstract
Even though the survival and proliferation stages of cancer cells that have newly settled at a metastatic site are the rate-limiting stages and the most promising targets for drugs, there is a lack of models of the earliest stage of metastasis formation. A method for modeling breast cancer liver metastasis is described here: a stage of transition of a differentiated tumor cell into a cell actively proliferating in a three-dimensional (3D) liver spheroid. Opposite to existing heterocellular 3D models of metastases, the protocol allows modeling the initial stage of liver colonization by metastatic cells, the so-called “micrometastases.” The method includes obtaining a line of fluorescent tumor cells, fluorescence-activated sorting of differentiated cells, preparing a single-cell suspension of liver cells, forming a liver spheroid in an agarose mold, inducing the tumor cell dedifferentiation and proliferation using IL-6, and intravital microscopy of spheroids, with subsequent processing and analysis of fluorescent images in the ImageJ software. The performance of the proposed model was demonstrated using microRNA therapeutics. The ability of a combination of microRNAs to suppress the transition of micrometastasis to macrometastasis in the 3D liver spheroid was confirmed by an immunofluorescent assay of spheroid sections and transcriptome analysis.
Key features
• The method introduces a 3D model of liver micrometastasis formation using differentiated tumor cells.
• The 3D spheroid consists of all the main types of normal liver cells and better reproduces the microenvironment.
• The method allows one to evaluate the effectiveness of a drug that blocks the transition of micrometastases to macrometastases.
• The model is optimal for studying RNA-based therapeutic agents, as well as prodrugs that require metabolism in the liver for activation.
Keywords: Spheroid (类球体)Graphical overview
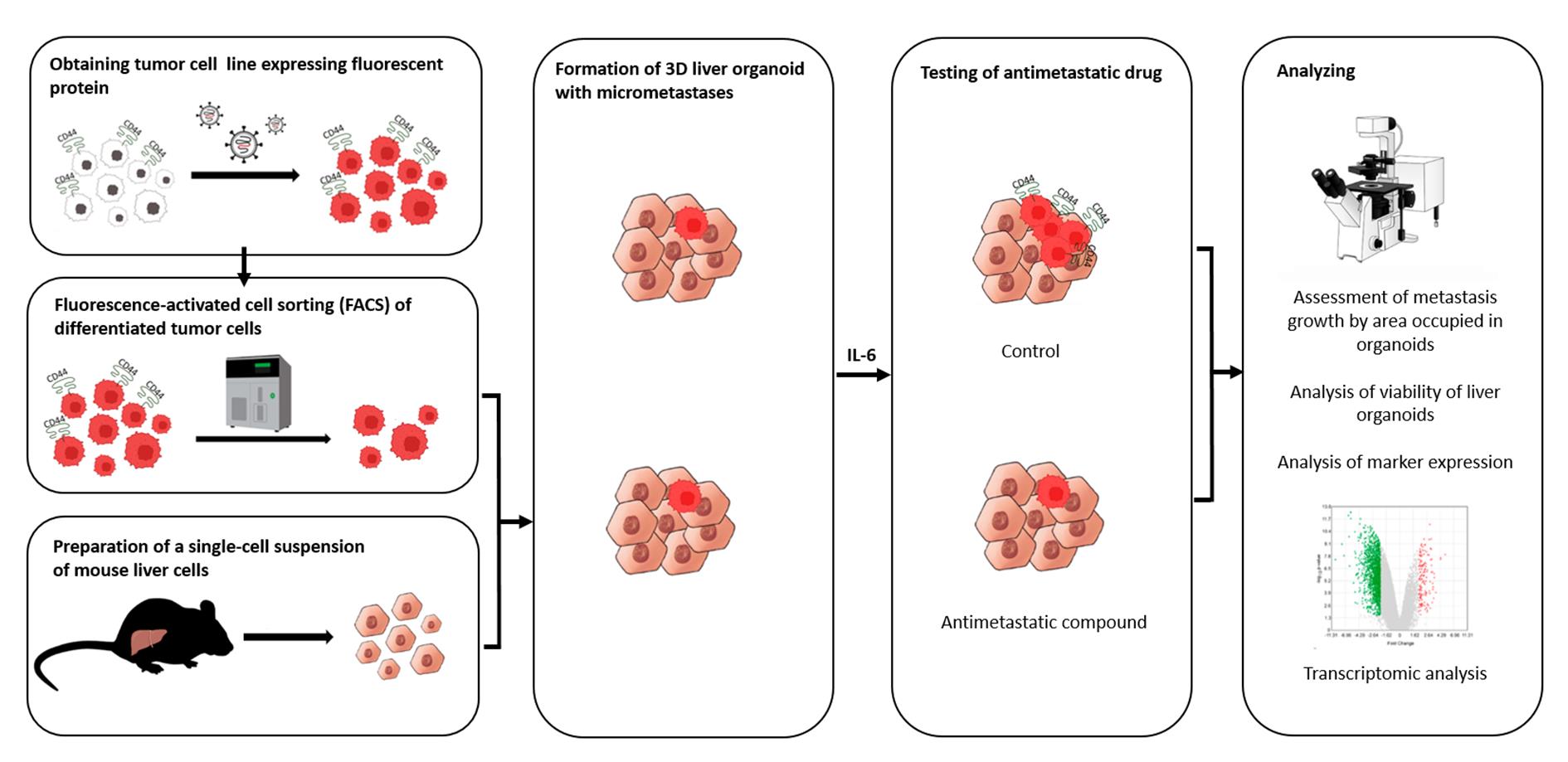
Background
Metastases are some of the main causes of mortality in cancer patients. In this regard, the development of effective methods for studying antimetastatic therapies is an important scientific task. After getting into a distant organ, cancer cells find themselves in a new microenvironment, and those that survive give rise to a secondary tumor, whose biology differs from that of the primary tumor. To effectively prevent the formation of secondary tumors, it is necessary to devise specific therapeutic strategies and agents [1] that are aimed at suppressing key stages of metastasis.
According to the current understanding of this phenomenon, a metastatic cascade is a multistage process that includes the following stages: invasion and intravasation, survival in the circulation and dissemination, arrest in a distant organ and extravasation, formation of micrometastasis and dormancy, escape of dormancy, and dedifferentiation, which end in macrometastasis growth [2]. The action of the vast majority of antimetastatic drugs developed to date is aimed at preventing the invasiveness and migration of tumor cells [3]. Nonetheless, hematogenous dissemination of cancer cells is often a very early event in tumor progression [4]. Moreover, metastasis, in particular in breast cancer, can remain undetectable for months to decades after initial diagnosis and treatment [5]. Thus, survival and proliferation of cancer cells that have newly settled at a metastatic site (micrometastasis) are the rate-limiting stage [6] and the most promising targets for antimetastatic drugs.
Drugs acting on a secondary tumor can be designed to inhibit mesenchymal-epithelial transition, prolong the dormant state [7], block protective autophagy [8], or suppress the dedifferentiation of tumor cells into stem-like cells [9]. Accordingly, the development of micrometastasis models for evaluating the effectiveness of new antimetastatic drugs is in demand.
Two-dimensional (2D) cell cultures, represented by a monolayer of more than 1,000 tumor cells, are not able to adequately model metastatic colonization of a distant organ by one or several disseminated tumor cells. Therefore, at present, animal models are used to test antimetastatic agents and biomedical technologies. Among them, mouse models are the main research tool [10], and the latest approaches devised in recent years make it possible to examine the behavior of individual cells in a metastatic niche in vivo [11].
Nonetheless, the extremely high attrition rate of drugs already in the first phase of clinical trials underscores the poor transferability of research results obtained in animal models to humans. Preclinical models are constantly being improved to more fully reflect the processes occurring in humans, for example, through xenotransplantation of a human tissue, creation of genetically engineered animals carrying mutant alleles/oncogenes, or humanized animals that more accurately model a human immune response [12]; however, some crucial features of human biology cannot be reproduced in other mammals, even in nonhuman primates [13].
This problem is especially serious during the determination of the biological activity of gene- and RNA-targeted drugs. Genomic differences caused by phenomena such as retrotranspositions, gene amplification or deletion, genomic rearrangements, differences in coding and noncoding sequences, or differences in expression regulation mediated by epigenetic mechanisms collectively are responsible for species-specificity of a gene expression profile [14]. For instance, only 80% of human protein-coding genes and 72% of murine ones have a one-to-one orthologous relation (~15,000 genes). The remaining 20%–30% of protein-coding genes are either in one-to-many or many-to-many orthologous relations and are members of gene families that have undergone species-specific expansion or contraction or contain species-specific open reading frames (ORFs). Even more severe divergences are characteristic of noncoding RNA sequences. For example, almost 3,000 and 2,000 microRNAs (miRNAs, miRs) are annotated in the human genome and mouse genome, respectively, but only a small proportion of them (300 miRNAs) have a specific ortholog [14]. Moreover, the effects of small noncoding RNAs are context dependent [e.g., the presence (in the cell) of sets of long noncoding RNAs, circular RNAs, and RNA-binding, RNA-modifying, and RNA-editing proteins]. As a consequence, for example, the same miRNA can act as a tumor suppressor in one situation and as an oncogene in another [15]. A comparative transcriptomic analysis showed that many biological processes are subject to human-specific regulation and can be extrapolated from mice to humans only with caution [16]. Additional serious limitations of mouse models are low tropism, i.e., low probability of metastasis formation in a given organ, and a long latency period [10,17]. In general, the establishment of animal models is expensive, time-consuming, and laborious, which makes them extremely inconvenient at initial stages of drug development and of evaluation of a drug’s specific activity.
In this regard, more and more attention is given to the creation of in vitro 3D models [17,18]. The liver is one of the most common sites of cancer metastasis [19]. For instance, metastases of breast cancer to the liver occur in 32%–35% of patients with cancer recurrence [20]. On the other hand, there are a few models of breast cancer metastasis to the liver [18]. In recent years, models based on microfluidic systems—microphysiological systems or organ-on-a-chip—have been actively designed. They allow for simulating the process of tumor cell extravasation and colonization of a secondary site by tumor cells, for example, breast cancer cells [21,22] and lung cancer cells [23]. Despite high research potential, successful reproduction of experimental models in an organ-on-a-chip system is still available only to a small number of laboratories, requires special equipment, and is quite expensive.
In this article, we describe our protocol for modeling the earliest stage of secondary-lesion formation, namely, the transition of a differentiated tumor cell to a cell actively proliferating in a three-dimensional (3D) liver organoid. The model is based on obtaining a line of fluorescent tumor cells, isolating a population of differentiated cells by sorting, incorporating them into a liver organoid, inducing the transition of micrometastasis to macrometastasis by means of IL-6, and analyzing the organoids by intravital microscopy with subsequent processing and analysis of fluorescent images in the ImageJ software.
Validation of the proposed model was performed using miRNA therapeutics. Recently, we proposed a strategy for metastasis prevention with the help of a combination of three miRNAs that inhibit stemness genes, block the dedifferentiation of cancer cells in a metastatic niche, and prevent macrometastasis formation. In the original work, to confirm the efficacy of the combination of miRNAs, via intravenous administration of miRNAs in liposomal form, we conducted a series of experiments indicating the suppression of stemness gene expression, inhibition of mammosphere formation in vitro, and the prevention of metastasis in mice carrying experimental tumors [9]. In the present article, we confirm the ability of the combination of miRNAs to suppress the transition of micrometastasis to macrometastasis in our proposed model of breast cancer metastasis in a 3D liver organoid.
Materials and reagents
Biological materials
1. Human infiltrating ductal carcinoma of the breast, T47D cells (Bioresource Collection at N.N. Blokhin National Medical Research Center of Oncology, Moscow, Russia, ATCC HTB-133)
2. Human embryonic kidney 293T cells (ATCC, CRL-3216)
3. Human monocyte-like THP-1 cells (ATCC, TIB-202)
4. Human hepatocellular carcinoma HepG2 cells (State Research Center of Virology and Biotechnology VECTOR, 353 Koltsovo, Russia, ATCC, HB-8065)
5. Packaging plasmid pMDLg/pRRE#54 (Addgene plasmid #12251)
6. Packaging plasmid pRSV-Rev (Addgene plasmid #12253)
7. Envelope plasmid pMD2.G (Addgene plasmid #12259)
8. Lenti-gRNA-RFP plasmid (a gift from Dr. Maxim N. Karagyaur, Institute of Regenerative Medicine, Lomonosov Moscow State University, Russia)
9. C57BL/6 mice (SPF vivarium of the Institute of Cytology and Genetics, the Siberian Branch of the Russian Academy of Sciences, Russia); 8–12-week-old male mice were used throughout the experiments
Reagents
1. Poly-L-lysine hydrobromide (Paneco, catalog number: Ф069)
2. Phosphate buffered saline (PBS) (Sigma, catalog number: P4417)
3. Dulbecco's modified Eagle's medium/nutrient Ham's mixture F-12 (DMEM/F-12) (Gibco, catalog number: 11320033)
4. RPMI-1640 medium (Gibco, catalog number: 31870025)
5. Fetal bovine serum (FBS) (Gibco, catalog number: 10091148)
6. Heat-inactivated FBS (Gibco, catalog number: A5670502)
7. GlutaMAX, 100× (Gibco, catalog number: 35050061)
8. Antibiotic-antimycotic, 100× (Sigma, catalog number: A5955)
9. Collagenase, Type II (Paneco, catalog number: 2275)
10. Potassium bicarbonate (KHCO3) (Sigma, catalog number: 237205)
11. Ammonium chloride (NH4Cl) (Sigma, catalog number: А4514)
12. Ethylenediaminetetraacetic acid disodium salt dehydrate (Na2EDTA·2H2O) (Helicon, catalog number: Am-O105B-0.5)
13. William's E medium (Gibco, United Kingdom, catalog number: 12551032)
14. Dexamethasone (KPKA, catalog number: ЛП-006755)
15. Insulin-transferrin-selenium, 100× (Biolot, catalog number: 1.2.006)
16. Agarose (Helicon, catalog number: B-5000-0.5)
17. Calcein AM (Invitrogen, catalog number: C1430)
18. Dimethyl sulfoxide (DMSO) (Paneco, catalog number: Ф135)
19. Polybrene (Sigma, catalog number: 107689)
20. TrypLE Express Enzyme (1×), no phenol red (Gibco, catalog number: 12604021)
21. Metafectene (Biontex, catalog number: T040-1.0)
22. Isoflurane (Karizoo, catalog number: ЛП-006558)
23. Accutase (Stemcell Technologies, catalog number: 07922)
24. Fluorescein isothiocyanate (FITC)-conjugated anti-human CD44 antibody (clone BJ18, BioLegend, catalog number: 338804)
25. Allophycocyanin (APC)-conjugated anti-human CD24 antibody (clone ML5) (BioLegend, catalog number: 311118)
26. Interleukin 6 (IL-6) (Abcam, catalog number: ab9627)
27. Paraformaldehyde (Sigma, catalog number: P6148-500G)
28. Sucrose (GERBU, catalog number: 1331)
29. Optimal cutting temperature (OCT) Tissue Tek compound (Sakura, catalog number: 4583)
30. Isopentane (Fisher Chemical, catalog number: P/1030/08)
31. Bovine serum albumin (BSA) (Sigma, catalog number: A2153-100G)
32. Fluoroshield mounting medium with DAPI (Abcam, catalog number: ab104139)
33. RNA later (Qiagen, catalog number: 1018087)
34. RNeasy Plus Mini kit (Qiagen, catalog number: 74134)
35. Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) (pH 8.0) (Helicon, catalog number: Am-0234-0.5)
36. Sodium chloride (NaCl) (Helicon, catalog number: H-1401-1.0)
37. Hanks' balanced salt solution (HBSS), calcium, magnesium, no phenol red (Gibco, catalog number: 14025092)
38. RNA oligonucleotides (Syntol, Russia)
39. Cyclophosphamide (Deko, catalog number: ЛС-001048)
Solutions
1. Coating of wells with poly-L-lysine (see Recipes)
2. DMEM/F-12 or RPMI 1640 medium (see Recipes)
3. Collagenase type II (see Recipes)
4. Ammonium-chloride-potassium (ACK) buffer (see Recipes)
5. William’s E medium (see Recipes)
6. Agarose solution (see Recipes)
7. Agarose molds (see Recipes)
8. Calcein AM solution (see Recipes)
9. Polybrene solution (see Recipes)
10. Mixture of miRNAs (see Recipes)
Recipes
1. Coating of wells with poly-L-lysine
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Water | 100% | 50 mL |
| Poly-L-lysine hydrobromide | 0.1 mg/mL | 5 mg |
Prepare a 0.1 mg/mL solution in water. Store at 4 °C. To coat the bottom of the wells of a 6-well plate, add 500 μL of the solution into each well and incubate for 1 h in a CO2 incubator. At the end of the incubation, wash three times with 1 mL of 1× PBS. Use immediately or let it dry for future use. Store at 4 °C for no more than 4 weeks.
2. DMEM/F-12 or RPMI 1640 medium
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F-12 or RPMI 1640 medium | 88% | 440 mL |
| FBS | 10% | 50 mL |
| GlutaMAX | 1% | 5 mL |
| Antibiotic-antimycotic solution (optional) | 1% | 5 mL |
To the DMEM/F-12 or RPMI 1640 medium, add 10% of FBS, 1× GlutaMAX, and an antibiotic-antimycotic solution (optional). In case of cultivation with an antibiotic-antimycotic, penicillin at 10 U/mL, streptomycin at 10.0 μg/mL, and amphotericin B at 25 μg/mL can be added. Store at 4 °C for no more than 4 weeks.
3. Collagenase type II
| Reagent | Final concentration | Volume |
|---|---|---|
| HBSS | 10% | 1 mL |
| Collagenase type IV (powder) | 0.1 mg/mL | 1 mg |
| DMEM/F-12 | 90% | 9 mL |
Prepare a stock solution of type II collagenase (125 U/mg) with a concentration of 1 mg/mL: Dilute 1 mg of collagenase powder in 1 mL of HBSS. Pass it through a 0.22-μm filter. To prepare a working solution of the collagenase, mix 9 mL of the complete DMEM/F-12 medium and 1 mL of the collagenase stock solution. Prepare the stock and working solutions of collagenase immediately before use.
4. Ammonium-chloride-potassium (ACK) buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| KHCO3 | 9.99 mM | 1 g |
| NH4Cl | 154.42 mM | 8.26 g |
| Na2EDTA·2H2O | 0.10 mM | 37.2 mg |
| H2O | to 1 L |
Dissolve 1 g of KHCO3, 8.26 g of NH4Cl, and 37.2 mg of Na2EDTA·2H2O in 800 mL of distilled deionized water. Measure pH and adjust it to 7.2–7.4 if necessary. Bring volume to 1 L with water and pass the solution through a 0.22-μm filter. Store at 4 °C for no more than 6 months.
5. William’s E medium
| Reagent | Final concentration | Volume |
|---|---|---|
| William’s E medium | 86% | 430 mL |
| FBS | 10% | 50 mL |
| GlutaMAX | 1% | 5 mL |
| Dexamethasone (4 mg/mL) | 1% | 5 mL |
| Insulin-transferrin-selenium solution | 1% | 5 mL |
| Antibiotic-antimycotic solution | 1% | 5 mL |
To William’s E medium, add 10% of heat-inactivated FBS, 1× GlutaMAX, dexamethasone to a final concentration of 40 μg/mL, a 1× insulin-transferrin-selenium solution (final concentration: 10 mg/L insulin, 5.5 mg/L transferrin, and 67 μg/L sodium selenite), and an antibiotic-antimycotic solution (e.g., penicillin 10 U/mL, streptomycin 10 μg/mL, and amphotericin B 25 μg/mL). Store at 4 °C for no more than 2 weeks.
6. Agarose solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Agarose | 2% | 2 g |
| 1× PBS | 98% | 100 mL |
Dissolve 2 g of agarose in 100 mL of 1× PBS. Sterilize by autoclaving for 20 min at 15 psi (1.05 kg/cm2) on a liquid cycle. Store at room temperature for no more than 6 months.
7. Agarose molds
Heat the agarose solution in a water bath to 95 °C or in a microwave oven (in pulse mode) until boiling. To form 81 wells, pour 500 μL of hot agarose into silicone molds (3D Petri Dish; MicroTissues Inc.). After the agarose mold solidifies (after ~5 min), remove it from the mold and place it in a well of a 12-well plate. Add 1 mL of complete William’s medium (Recipe 5) into each well and incubate for at least 10–15 min. Immediately before use, remove the complete medium from the well and from the agarose mold. Agarose molds can be prepared either on the day of the experiment or in advance. If the molds are prepared in advance, then after removing them from the silicone paternal mold, they must be filled with 1× PBS supplemented with 2× concentration of antibiotics. Seal the plate (or Petri dish) containing the molds air-tightly with parafilm and store at 4 °C for no more than a week.
8. Calcein AM solution
| Reagent | Final concentration | Volume |
|---|---|---|
| Calcein AM stock solution (1 mg/mL, 1 mM) | 2 μM | 1 μL |
| 1× PBS | 0.5 mL |
To obtain a stock solution, dilute this reagent in DMSO to a concentration of 1 mg/mL (1 mM). Store the resulting stock solution at -20 °C. The working solution of calcein AM is prepared immediately before use by diluting to a concentration of 2 μM. To this end, dilute the stock solution 1:500 with HBSS or a medium without added FBS.
9. Polybrene solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Polybrene | 10 mg/mL | 10 mg |
| H2O | 1 mL |
Dilute polybrene to 10 mg/mL with water. Pass it through a 0.22-μm filter. Store at 4 °C.
10. Mixture of miRNAs
miRNA mimics were prepared using a protocol described in [9]. The RNA oligonucleotide sequences are provided in Table S1. Three miRNA mimics (miR-195-5p, miR-520a, and miR-630) in 10 mM Tris (pH 8.0), 50 mM NaCl, and 0.1 mM Na2EDTA·2H2O were mixed at equimolar concentrations at a final concentration of each miRNA of 50 nM. For testing, the microRNA mixture and Metafectene PRO were mixed in a 5:1 ratio according to the manufacturer's protocol and transferred dropwise to spheroids in individual wells of a 12-well plate.
Laboratory supplies
1. Cell culture plates, 6-well, tissue culture treated (SPL Lifesciences, catalog number: 30006)
2. Cell culture plates, 12-well, tissue culture treated (SPL Lifesciences, catalog number: 30012)
3. T25 flask, tissue culture treated (SPL Lifesciences, catalog number: 70075)
4. T75 flask, tissue culture treated (SPL Lifesciences, catalog number: 70025)
5. 5 mL serological pipettes (SPL Lifesciences, catalog number: 95005)
6. 10 mL serological pipettes (SPL Lifesciences, catalog number: 95010)
7. 25 mL serological pipettes (SPL Lifesciences, catalog number: 91025)
8. 10 μL pipette tips (GenFollower, catalog number: FTB10-10)
9. 200 μL pipette tips (GenFollower, catalog number: TVB200YE)
10. 1,000 μL pipette tips (GenFollower, catalog number: TB1000-10)
11. Microcentrifuge tubes, 1.5 mL (GenFollower, catalog number: LMTB015)
12. Centrifuge tubes, 15 mL (SPL Lifesciences, catalog number: 50015)
13. Centrifuge tubes, 50 mL (SPL Lifesciences, catalog number: 70050)
14. Cell strainer, 100 μm (SPL Lifesciences, catalog number: 93100)
15. 3D Petri dish micro-mold spheroids, 9 × 9 array (MicroTissues, catalog number: Z764019)
16. Syringe filter, 0.45 μm (GVS Abluo, catalog number: FJ25BSCPS004AP01)
17. Syringe filter unit, PES, 0.22 μm (GVS Abluo, catalog number: FJ25BSPSA002AL01)
18. ClariomTM S assay platform (Affymetrix, catalog number: 902927)
19. Microscope Polylisine slides (Thermo Scientific, catalog number: J2800AMNZ)
20. CountessTM cell counting chamber slides (Invitrogen, catalog number: C10228)
Equipment
1. Class II biological safety cabinet (Lamsystems, model: 1R-B.001-15)
2. Laboratory centrifuge with cooling (Nuve, model: NF400R)
3. pH meter (MettlerToledo, model: G2S)
4. CO2 incubator (Sanyo, model: МСО15АС)
5. Autoclave (Tuttnauer, model: TUT 2540)
6. Stirred water bath (Biosan, model: WB-4MS)
7. Microwave oven
8. PCR cabinet (Lamsystems, model: 1R-F.004-10)
9. Thermal cycler (Applied Biosystems, model: 2720)
10. Porkka ice flake machines (Porkka, model: 45А)
11. Cryogenic vessel (Thermo, model: Biocane 47 СK509Х4)
12. Flow cytometer Cytoflex (Beckman Coulter, model: B5-R3-V5)
13. Incubating rocking platform shaker (Russia, model: KТ 103)
14. Cryostat (Thermo Scientific, model: HM525 NX)
15. Centrifuge (Eppendorf, model: MiniSpin Plus)
16. Classic anesthesia system (Braintree Scientific, model: EZ-7000-320)
17. Automated Cell Counter Countess II FL (Thermo, model: Countess II FL)
18. Fluorescent microscope equipment with DFC9000 GT sCMOS (Leica, model: DMi8)
19. Confocal laser scanning microscope (Nexcope, model: NCF1000)
20. Cell sorter FuTech (Shanghai Future Technology Co., Ltd., model: SE309)
21. NanoDrop-2000 spectrophotometer (Thermo Scientific, model: ND-2000)
22. TapeStation instrument for capillary electrophoresis (Agilent Technologies, model: 4150)
23. GeneChip System 3000 (Affymetrix, model: 00-0218)
Software and datasets
1. Image-processing freeware ImageJ (Fiji) [24]
2. Transcriptome Analysis Console (TAC) software (version 4.0)
Procedure
文章信息
稿件历史记录
提交日期: Jul 2, 2025
接收日期: Aug 20, 2025
在线发布日期: Sep 2, 2025
出版日期: Sep 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Nevskaya, K. V., Pershina, A. G., Efimova, L. V., Sukhinina, E. V., Kozlova, P. K., Ryzhkova, A. Y., Hmelevskaya, E. S., Ibragimova, M. K., Tsydenova, I. A., Litviakov, N. V. and Udut, E. V. (2025). A Model of Breast Cancer Micrometastasis in a Three-Dimensional (3D) Liver Spheroid for Testing an Antimetastatic Therapy. Bio-protocol 15(18): e5454. DOI: 10.21769/BioProtoc.5454.
分类
癌症生物学 > 侵袭和转移 > 动物模型
癌症生物学 > 通用技术 > 药物发现和分析
癌症生物学 > 微环境
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









