- EN - English
- CN - 中文
Genetic Engineering of Humanized Telomere Mice
人源化端粒小鼠的基因工程构建
发布: 2025年09月20日第15卷第18期 DOI: 10.21769/BioProtoc.5445 浏览次数: 2587
评审: Alka MehraAnonymous reviewer(s)
Abstract
Telomere shortening is a hallmark of human aging, and telomerase regulation plays a critical role in cellular proliferation and replicative senescence. In human cells, telomere length imposes a limit on proliferative potential, a phenomenon known as the Hayflick limit. However, species-specific differences in telomere dynamics and telomerase regulation between humans and mice present challenges to using mice as accurate models for human telomere-related research. To address this limitation, we engineered a humanized telomerase gene (hmTert) in mice by replacing the non-coding sequences within the mouse Tert locus (mTert) with corresponding regulatory sequences from the human TERT gene. Breeding of these genetically modified mice resulted in progressive telomere shortening over successive generations, ultimately reaching human-like lengths (below 10 kb). This protocol outlines the development of this humanized telomere mouse model, referred to as HuT mice, offering a robust platform for studying human telomere biology and aging-related diseases.
Key features
• This protocol describes methods to increase the success rates of knocking in large genomic fragments (~47 kb) by integrating CRISPR-Cas9 with homologous recombination.
• It enables precise engineering of a humanized telomerase gene (hmTert), faithfully recapitulating human TERT regulation and telomere length dynamics in mice.
Keywords: Genetic engineering (基因工程)Graphical overview
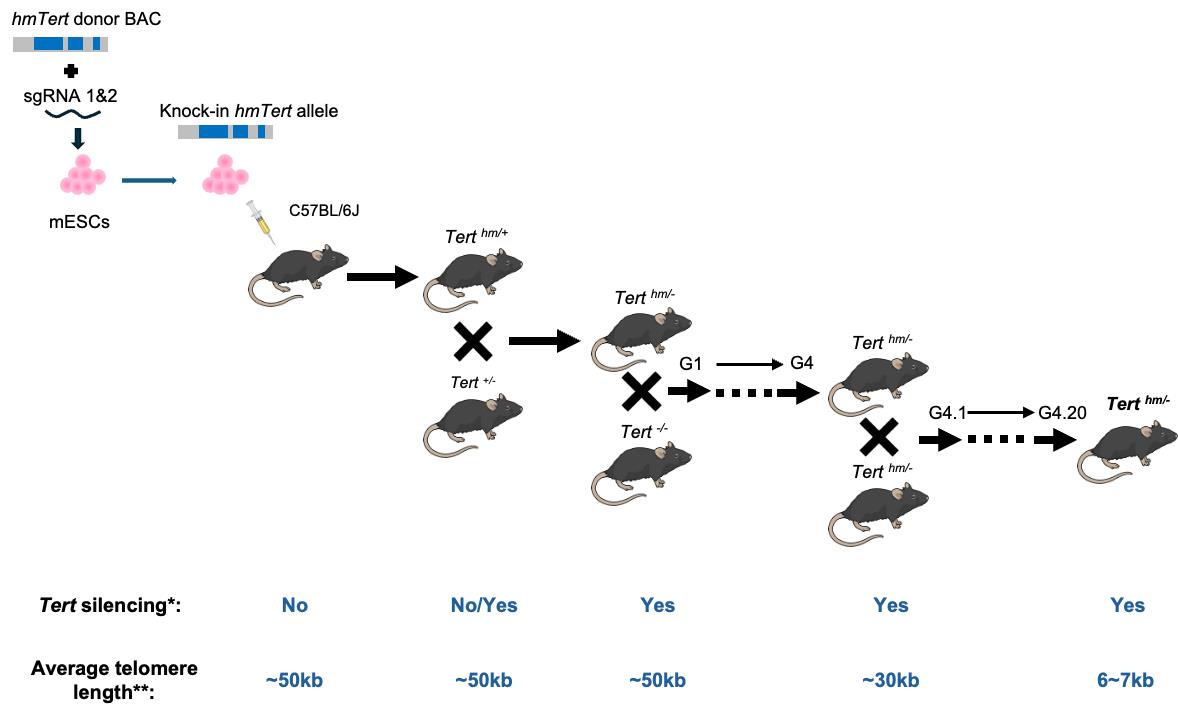
*No indicates that Tert expression is found in many mouse adult tissues; Yes indicates that Tert mRNA is not detected in all adult tissues except for testis, ovary, and thymus.
**Telomere length is measured in splenocytes by Flow-FISH.
Background
Telomeres, repetitive sequences at chromosome ends, are essential for maintaining genomic stability. In most human somatic cells, telomeres progressively shorten with each cell division, a process counteracted by the telomerase enzyme complex, which consists of the TERT protein and TERC RNA subunits. Most human adult tissues contain low or no telomerase, and telomere shortening eventually triggers cell cycle arrest, leading to replicative senescence or the Hayflick limit. Telomerase elongates telomeres, delaying senescence. However, telomere homeostasis and telomerase regulation differ markedly between species. For instance, while telomere lengths in human tissues typically range from 10 to 15 kb, laboratory mouse strains, such as C57BL/6, often exhibit telomeres over ~50 kb. Moreover, telomerase remains active in most adult mouse tissues, unlike in humans, where it is largely repressed [1]. As a result, mouse cells do not exhibit replicative senescence or the Hayflick limit.
Telomerase knockout (KO) mice, including those deficient in Tert or Terc, exhibit limited viability by the sixth generation. Despite lacking telomerase, these mice maintain average telomere lengths exceeding 25 kb—substantially longer than those in humans [2]. Importantly, early-generation telomerase-null mice do not display overt phenotypic abnormalities [3], suggesting that mice have considerable telomere reserves capable of sustaining multiple generations before critical shortening occurs. These species-specific differences significantly limit the usefulness of conventional mice as models for studying human telomere biology and aging-related diseases.
Our laboratory focuses on the regulation of the TERT gene, which encodes the rate-limiting catalytic subunit of telomerase. We have identified key regulatory regions within the human TERT gene—including the 5′ intergenic region, intron 2, and intron 6—that modulate TERT expression [4]. Replacing those genetic contexts on the mouse locus with human counterparts disrupted Tert repression in differentiated mouse ESCs [5]. To investigate the role of these elements in vivo, we engineered a humanized telomerase gene (hmTert) in mouse embryonic stem cells (mESCs) by replacing the corresponding non-coding regions in the mouse Tert locus with a 47 kb hybrid sequence from a bacterial artificial chromosome (BAC) containing the human regulatory elements [6–8]. Initial attempts to introduce this large fragment via homologous recombination were hindered by low efficiency and incomplete integration. To overcome this, we employed CRISPR-Cas9 technology to enhance homologous recombination efficiency, increasing the success rate from 0.05% to 11%, in mESCs [8]. The hmTert gene in mESCs accurately recapitulated human TERT regulation during in vitro differentiation [5]. We subsequently established a mouse strain carrying germline hmTert alleles. Investigation of these mice confirmed human-like Tert regulatory patterns. With successive intercrossing of heterozygous mice, telomere lengths gradually shortened across generations, eventually reaching a human-like length of less than 10 kb. Despite their much shorter telomeres, Terth/h and Terth/- mice maintained normal body weight and tissue homeostasis. These mice, which exhibit physiologically relevant telomerase regulation, serve as a powerful model for studying human telomere biology, aging, and associated diseases.
This protocol outlines the key steps for generating and validating the humanized telomere mouse model, known as HuT mice.
Materials and reagents
Biological materials
1. Mouse embryonic stem cells G4 [9]
2. Drug-resistant (DR4) feeder cells: mouse embryonic fibroblast cells derived from E13.5–14.5 embryos and treated as previously described [7]
3. pSpCas9 (BB)‐2A‐GFP (PX458, Addgene #48138), a gift from Feng Zhang [10]
Reagents
1. 7-AAD (Thermo Fisher, catalog number: 00-6993-50)
2. Agarose (Invitrogen, catalog number: 16500-500)
3. LB broth (Affymetrix, catalog number :75852)
4. Agar (VWR, catalog number: J637)
5. DMEM medium (HyClone, catalog number: SH30243)
6. Penicillin/Streptomycin solution 100× (Gemini, catalog number: 400-109-100)
7. NEAA 100× (HyClone, catalog number: SH30238.01)
8. Fetal bovine serum (FBS) (Atlanta, catalog number: S12450)
9. RPMI medium (HyClone, catalog number: SH30027.LS)
10. Lipofectamine 2000 (Thermo Fisher, catalog number: 11668027)
11. Ladderman DNA Labeling kit (Takara, catalog number: 6046)
12. Isopropanol (MP Biomedical, catalog number: 0219400690)
13. Ethanol absolute, pure (200 Proof) (Avantor, catalog number: 71001-754)
14. Chloroform (MP Biomedical, catalog number: 0219400225)
15. TRI reagent (Ambion, catalog number: 155960118)
16. PowerUpTM SYBRTM Green Master Mix for qPCR (Thermo Fisher, catalog number: A25778)
17. Deoxynucleotide (dNTP) solution mix (NEB, catalog number: N0447)
18. QIAEX II Gel Extraction kit (Qiagen, catalog number: 20021)
19. SuperScriptTM III reverse transcriptase (Thermo Fisher, catalog number: 18080044)
20. TaKaRa LA Taq® DNA polymerase (Takara, catalog number: RR002B)
21. Reprobing charged Nylon (GVS, catalog number: 1226556)
22. Restriction digestion enzyme: all restriction enzymes were purchased from New England Biolabs (NEB)
23. NEB® 5-alpha Competent E. coli (NEB, catalog number: C2987H)
24. Taq DNA polymerase with ThermoPol® buffer (NEB, catalog number: M0267L)
25. T4 polynucleotide kinase (NEB, catalog number: M0201S)
26. T4 DNA ligase (NEB, catalog number: M0202L)
27. TelC-FAM probe (PNA Bio, catalog number: F1001): a PNA probe for leading stand of telomere sequences (CCCTAA)3 conjugated with FAM
28. GenEluteTM Plasmid Miniprep kit (Sigma, catalog number: PLN70)
29. Wizard® Genomic DNA Purification kit (Promega, catalog number: A1125)
30. 32P-dCTP (PerkinElmer, catalog number: BLU513H250UC)
31. Ampicillin sodium salt (Fisher Scientific, catalog number: BP1760-5)
32. Puromycin dihydrochloride (Sigma, catalog number: P8833)
33. Ganciclovir (GCV) (Sigma, catalog number: SML2346)
34. Roche blocking reagent (Roche, catalog number: 11096176001)
35. Trypsin solution (HyClone, catalog number: SH30042)
36. GlutaMaxTM supplement (Gibco, catalog number: 35050061)
37. LIF recombinant mouse protein (Gibco, catalog number: A35935)
38. β-Mercaptoethanol (Sigma, catalog number: M6250)
Solutions
1. Ampicillin solution (see Recipes)
2. 50 mg/mL puromycin (see Recipes)
3. 10 mM GCV (see Recipes)
4. 10% blocking buffer (see Recipes)
5. LB medium (see Recipes)
6. 0.025% trypsin solution (see Recipes)
7. mESCs culture medium (see Recipes)
8. 0.5× TBE (see Recipes)
9. Denature buffer (see Recipes)
10. Neutralization buffer (see Recipes)
11. Church and Gilbert’s hybridization buffer (Church buffer) (see Recipes)
12. 20× SSC (see Recipes)
13. DNA wash solution (see Recipes)
14. Hank's balanced salt solution (HBSS) (see Recipes)
15. PBS (see Recipes)
16. Red blood cell (RBC) lysis buffer (see Recipes)
17. Hybridization buffer (see Recipes)
18. Wash solution I (see Recipes)
19. Wash solution II (see Recipes)
20. Maleic acid buffer (see Recipes)
Recipes
1. Ampicillin solution
A stock solution (100 mg/mL) is prepared by dissolving 100 g of ampicillin sodium salt into 10 mL of Milli-Q water and filtering it through a 0.22 μm filter. The stock solution is added to the LB medium at a final concentration of 100 μg/mL.
2. 50 mg/mL puromycin
A stock solution (50 mg/mL) is prepared by dissolving 0.5 g of puromycin dihydrochloride into 10 mL of Milli-Q water and filtering it through a 0.22 μm filter. The stock solution is added to the mESCs culture medium at a final concentration of 1.5 μg/mL.
3. 10 mM GCV
This stock solution is added to the mESCs culture medium at a final concentration of 50 μM.
4. 10% blocking buffer
Dissolve Roche blocking reagent in maleic acid buffer to a final concentration of 10% (w/v) by shaking and heating on a heating block.
5. LB medium
Dissolve 20 g of LB broth in 1 L of Milli-Q water and autoclave. Prior to autoclaving, add 15 g of agar to 1 L of LB medium to prepare the bacterial culture plate.
6. 0.025% Trypsin solution
Dilute 10 mL of 0.25% Trypsin in 40 mL of autoclaved PBS.
7. mESCs culture medium
| Reagent | Final concentration |
|---|---|
| FBS | 15% |
| GlutaMaxTM supplement (100×) | 1× |
| NEAA (100×) | 1× |
| LIF recombinant mouse protein | 10 ng/mL |
| β-mercaptoethanol | 55 µM |
| Penicillin/Streptomycin solution (100×) | 1× |
| DMEM medium | 85% |
8. 0.5× TBE
| Reagent | Final concentration |
|---|---|
| Tris base | 0.045 M |
| H3BO3 | 0.045 M |
| EDTA (pH 8.3) | 0.01 M |
9. Denature buffer
| Reagent | Final concentration |
|---|---|
| NaCl | 1.5 M |
| NaOH | 0.05 M |
10. Neutralization buffer
| Reagent | Final concentration |
|---|---|
| NaCl | 1.5 M |
| Tris base (pH 8.5) | 9.5 M |
11. Church and Gilbert’s hybridization buffer (Church buffer), pH 7.2
| Reagent | Final concentration |
|---|---|
| Na2HPO4 | 0.34 M |
| NaH2PO4 | 0.16 M |
| SDS | 7% (w/v) |
| EDTA | 0.001 M |
| BSA | 1% (w/v) |
12. 20× SSC, pH 7.0
| Reagent | Final concentration |
|---|---|
| NaCl | 3 M |
| Na3C6H5O7 | 0.3 M |
A series of SSC solutions used in the paper were diluted from 20× SSC with an appropriate volume of MilliQ water.
13. DNA wash solution
| Reagent | Final concentration |
|---|---|
| Na3C6H5O7 | 0.3 M |
| Ethanol | 10% (v/v) |
14. Hank's balanced salt solution (HBSS), pH 7.4
| Reagent | Final concentration |
|---|---|
| NaCl | 0.14 M |
| KCl | 0.005 M |
| CaCl2 | 0.001 M |
| MgSO4 | 0.0004 M |
| MgCl2 | 0.0005 M |
| Na2HPO4 | 0.0003 M |
| KH2PO4 | 0.0004 M |
| D-Glucose | 0.004 M |
| Na2CO3 | 0.004 M |
15. PBS, pH 7.2–7.4
| Reagent | Final concentration |
|---|---|
| NaCl | 0.137 M |
| KCl | 0.0027 M |
| Na2HPO4 | 0.01 M |
| KH2PO4 | 0.0018 M |
16. Red blood cell (RBC) lysis buffer, pH 7.4
| Reagent | Final concentration |
|---|---|
| NH4Cl | 155 M |
| KHCO3 | 10 M |
| EDTA | 0.1 M |
17. Hybridization buffer
| Reagent | Final concentration |
|---|---|
| Tris-HCl (pH 7.2) | 20 mM |
| Deionized formamide | 60% (v/v) |
| Blocking buffer | 0.5% (v/v) |
18. Wash solution I
| Reagent | Final concentration |
|---|---|
| Tris-HCl (pH 7.2) | 20 mM |
| Deionized formamide | 60% (v/v) |
| BSA | 0.1% (w/v) |
| Tween 20 | 0.1% (v/v) |
19. Wash solution II
| Reagent | Final concentration |
|---|---|
| BSA | 0.1% (w/v) |
| Tween 20 | 0.1% (v/v) |
| PBS | Add to final volume |
20. Maleic acid buffer (pH 7.5)
| Reagent | Final concentration |
|---|---|
| Maleic acid | 0.1 M |
| NaCl | 0.15 M |
Laboratory supplies
1. Cell culture multi-well plates (Greiner Bio-One, 12-well plate, 48-well pate, and 96-well plate)
2. 1.5 mL microcentrifuge tubes (Fisher Scientific, catalog number: 05-408-129)
3. 15 mL conical tubes (VWR, catalog number: 89039-658)
4. 50 mL conical tubes (VWR, catalog number: 21008-089)
5. 1 mL cryovials tubes (VWR, catalog number: I210-000001)
6. 5 mL serological pipettes (Greiner Bio-One, catalog number: 606107)
7. 10 mL serological pipettes (Greiner Bio-One, catalog number: 607107)
8. 0.1–10 μL pipette tips (Fisher Scientific, catalog number: 02-707-454)
9. 5–300 μL pipette tips (Fisher Scientific, catalog number: 02-707-447)
10. 100–1,250 μL pipette tips (Fisher Scientific, catalog number: 02-707-400)
11. 0.2 mL PCR tube (Corning, catalog number: PCR-02-A)
12. qRT-PCR plates (Thermo Fisher, catalog number: 4346907)
Equipment
1. PhosphorImager system (GE Healthcare)
2. CO2 incubator (Eppendorf, model: Galaxy 170S)
3. Incubator (VWR)
4. Incubator shaker (New Brunswick, model: I2400)
5. Water bath (Fisher Scientific, model: Isotemp215)
6. Centrifuge (Eppendorf, model: 5424R)
7. Laboratory centrifuge with rotors for 15- and 50-mL conical tubes (Eppendorf, model: 5804R)
8. Thermal cycler machine (Bio-Rad, model: C1000Touch)
9. Applied BiosystemsTM StepOnePlusTM Real-Time PCR System
10. CytoFLEX (Bechman Coulter)
11. Tissue cell culture hood
12. Liquid nitrogen (N2) tank
13. Freezer (-20 °C)
14. Refrigerator (2–8 °C)
15. Thermomixer (Eppendorf, model: 5436)
16. Hybridization incubator (Fisherbiotech)
17. UV-crossing link machine (Spectrolink, model: XL-1000)
18. pH meter (VWR, model: B10P)
19. Vortex (Fisher Scientific, catalog number: 12812)
Procedure
文章信息
稿件历史记录
提交日期: May 18, 2025
接收日期: Aug 3, 2025
在线发布日期: Aug 28, 2025
出版日期: Sep 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Zhang, F., Cheng, D., Porter, K. I., Wang, S. and Zhu, J. (2025). Genetic Engineering of Humanized Telomere Mice. Bio-protocol 15(18): e5445. DOI: 10.21769/BioProtoc.5445.
分类
发育生物学 > 基因组编辑 > 靶向整合
生物科学 > 生物技术 > CRISPR/Cas9
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












