- EN - English
- CN - 中文
Practical Guide to In Vitro Clonal Propagation of Nicotiana benthamiana Using Axillary Shoot Induction
本氏烟草的离体快繁与腋芽分枝培养方案
发布: 2025年09月20日第15卷第18期 DOI: 10.21769/BioProtoc.5443 浏览次数: 1167
评审: Venkatasalam ShanmugabalajiAnonymous reviewer(s)
Abstract
This protocol outlines a reliable method for the micropropagation of Nicotiana benthamiana using axillary shoot branching. Axillary shoot induction involves stimulating the outgrowth of dormant buds located at the leaf axils, allowing for the development of genetically stable shoots without callus formation or the use of exogenous plant growth regulators. Nodal explants are cultured on MS medium supplemented with kinetin and indole-3-butyric acid (IBA) to induce shoot formation. Isolated shoots are then transferred to hormone-free MS medium for rooting. This method is simple, reproducible, and supports rapid plant multiplication for downstream applications such as agroinfiltration or transient protein expression.
Key features
• Axillary branching-based micropropagation enables efficient regeneration of N. benthamiana from nodal explants, supporting a cyclic system for continuous in vitro plant supply [1].
• Rooting occurs on hormone-free medium, with even shoots as small as 0.5 mm readily forming roots, simplifying and accelerating the propagation process.
• Root formation begins within 7 days, including in transgenic lines that are typically difficult to root using conventional methods.
• Fully developed, rooted plants are ready within 4 weeks, ideal for transient expression assays, recombinant protein production, and secondary metabolite extraction.
Keywords: Micropropagation (离体快繁)Graphical overview
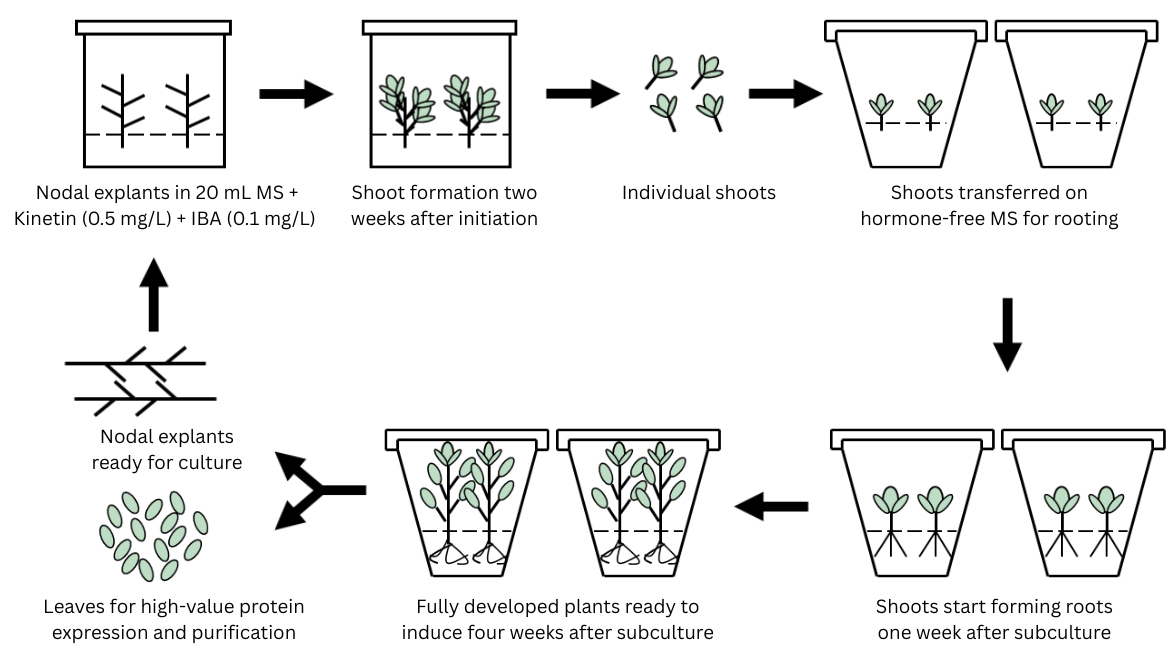
Schematic representation of the micropropagation workflow for N. benthamiana via axillary branching. The process includes nodal cutting culture, axillary shoot formation, shoot separation, rooting, and plantlet development for downstream use. This figure was created using Microsoft PowerPoint.
Background
Nicotiana benthamiana is a model plant widely used in plant molecular biology, particularly for transient gene expression, recombinant protein production, functional genomics research [2–4], and viral vector studies [5]. It is particularly amenable to Agrobacterium-mediated transformation and supports high levels of foreign gene expression. One of the most commonly used methods for transient gene expression in N. benthamiana is leaf infiltration. This technique involves introducing Agrobacterium tumefaciens carrying the gene of interest directly into the intercellular spaces of leaves. It allows for rapid and localized gene expression without the need for stable transformation. Leaf infiltration is simple, efficient, and highly reproducible, making it especially valuable for high-throughput functional genomics, protein localization, and recombinant protein production studies [4,6,7]. Its ease of use and adaptability make it a cornerstone method in plant biotechnology. One of its key advantages is the low level of interfering endogenous compounds, such as phenolics and proteases, which facilitates efficient recombinant protein purification and metabolite analysis [8,9].
To fully utilize N. benthamiana for high-throughput molecular studies or biomanufacturing applications, a reliable and efficient micropropagation system is essential. Ready access to uniform, healthy in vitro plants supports transient assays, stable transformation, and metabolite production. Although N. benthamiana is relatively easy to grow, propagation of transgenic lines and high-frequency regeneration systems remains a bottleneck. Efficient micropropagation techniques are essential for generating uniform plant material suitable for transformation and expression assays. Axillary branching offers a simple and hormone-responsive method for shoot multiplication, avoiding the complications associated with de novo regeneration. Continuous use of nodal segments from newly established in vitro plants enables a cyclic propagation system, ensuring a consistent and scalable supply of fully developed plants for downstream applications. Furthermore, since the rooting stage in this protocol does not require any auxins, it minimizes the risk of residual hormonal effects that could interfere with subsequent assays, such as transient gene expression. The following protocol is adapted from Deo et al. [1], enabling routine propagation of N. benthamiana on MS medium [10] under controlled conditions.
From practical experience, nodal cuttings from transgenic N. benthamiana are notoriously difficult to root, which limits shoot proliferation and leaf biomass, which are critical for recombinant protein extraction and purification. To address this limitation, the protocol described here utilizes a hormone-free micropropagation approach based on axillary shoot branching. Axillary shoot branching refers to the outgrowth of buds located at the junction between a leaf and stem (the axil), which can develop into new shoots under appropriate conditions. In tissue culture, these axillary buds can be stimulated to grow into genetically identical shoots without the need for dedifferentiation or callus formation, thereby reducing the risk of somaclonal variation and maintaining genetic stability. Efficient root induction from axillary shoots without the use of external auxins streamlines the micropropagation process. This rapid development supports a timely production of plants suitable for downstream applications such as transient gene expression and metabolite extraction. This system supports cyclic propagation, and using it, nearly 3 kg of in vitro leaves were harvested within six months, demonstrating scalability and robustness for molecular farming applications.
This protocol describes a simple, reproducible method for the mass propagation of N. benthamiana using axillary shoot branching, as illustrated in the Graphical overview. This method enables rapid multiplication and preparation of plants suitable for downstream applications such as agroinfiltration and recombinant protein expression.
Materials and reagents
Biological materials
1. N. benthamiana seeds (lab stock; originally obtained from the Centre for Tropical Crops and Biocommodities, now Centre for Agriculture and Bioeconomy, Queensland University of Technology). Researchers interested in acquiring seeds are advised to contact laboratories or research groups working with N. benthamiana for seed sharing, as commercial suppliers are limited
2. In vitro–cultured N. benthamiana plants (generated from lab stock seeds as described in the protocol)
Reagents
1. Murashige and Skoog (MS) basal medium powder (Sigma, catalog number: M5519)
2. Kinetin powder (Sigma, catalog number: K3378)
3. Indole-3-butyric acid (IBA) powder (Sigma, catalog number: I5386)
4. Sucrose (PhytoTech Labs, catalog number: S391)
5. Agar (or alternative gelling agent like phytagel) (Sigma, catalog number: A5054)
6. Commercial bleach (e.g., White KingTM 4% sodium hypochlorite) (from local supermarket)
7. Tween-20 (10%) (Sigma, catalog number: P9416)
8. Ethanol (70% and absolute) (Sigma, catalog number: 459844)
9. 1 M NaOH (Sigma, catalog number: 1091371000)
9. Sterile Milli-Q water for seed sterilization
10. Milli-Q water for hormone and media preparation
Solutions
1. IBA stock solution (see Recipes)
2. Kinetin stock solution (see Recipes)
3. MS basal medium without hormones (hormone-free MS) (see Recipes)
4. MS basal medium with hormones (MS1) (see Recipes)
5. Seed sterilization solution (see Recipes)
Recipes
Note: Be precise about the ingredients (e.g., buffer or media), quantities, and conditions established for your experiments. Note that the omission of minor details from recipes (e.g., type of water used or storage conditions) might lead to the failure of the experiment.
1. IBA stock solution (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| IBA powder | 1 mg/mL | 20 mg |
| Absolute ethanol | 70% | 14 mL |
| Milli-Q water | up to 20 mL | |
| Total | 20 mL |
Weigh 20 mg of IBA powder and transfer to a 50 mL Falcon tube. Dissolve in 14 mL of absolute ethanol. Make up the final volume to 20 mL with Milli-Q water. The final concentration of ethanol will be 70%. Filter sterilize using a 0.2 μm syringe filter. Aliquot 1 mL into sterile 1.5 mL Eppendorf tubes. Store at -20 °C. Use 100 μL per 1 L of MS medium for a final IBA concentration of 0.1 mg/L.
2. Kinetin stock solution (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Kinetin powder | 1 mg/mL | 20 mg |
| 1 M NaOH | 100 μL | |
| Milli-Q water | up to 20 mL | |
| Total | 20 mL |
Weigh 20 mg of kinetin powder and transfer to a 50 mL Falcon tube. Dissolve in a few drops of 1 M NaOH and make up the final volume to 20 mL with Milli-Q water. Filter sterilize using a 0.2 μm syringe filter. Aliquot 1 mL into sterile 1.5 mL Eppendorf tubes. Store at -20 °C. Use 500 μL per 1 L of MS medium for a 0.5 mg/L final concentration.
3. MS basal medium without hormones (hormone-free MS)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder | 4 g/L | 4 g |
| Sucrose | 30 g/L | 30 g |
| Agar | 7 g/L | 7 g |
| Milli-Q water | n/a | up to 1,000 mL |
| Total | 1,000 mL |
Dissolve MS powder in 700 mL Milli-Q water. Add sucrose and mix well. Adjust pH to 5.8 using 1 M NaOH. Make up the volume to 1 L. Add 7 g of agar. Autoclave at 121 °C at 15 psi for 15 min. Cool to 40–50 °C and pour 70 mL per sterile 250 mL culture container in a laminar airflow hood.
Storage tip: Autoclaved hormone-free MS medium can be stored in the dark at room temperature (23–24 °C) for up to 3–4 weeks without compromising effectiveness.
4. MS basal medium with hormones (MS1)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder | 4 g/L | 4 g |
| Sucrose | 30 g/L | 30 g |
| Agar | 7 g/L | 7 g |
| Kinetin stock | 0.5 mg/L | 500 μL |
| IBA stock | 0.1 mg/L | 100 μL |
| Milli-Q water | up to 1,000 mL | |
| Total | 1,000 mL |
Follow the same steps as for the hormone-free MS solution. After autoclaving and cooling to 40–50 °C, add 500 μL of kinetin stock (1 mg/mL) and 100 μL of IBA stock (1 mg/mL). Mix gently and pour 20 mL per sterile 120 mL culture container in a laminar airflow hood.
Storage tip: Hormone-supplemented MS medium is best used fresh or within 1–2 weeks when stored at room temperature (23–24 °C), as prolonged storage may reduce hormone activity.
Time-saving tip: Prepare hormone-free MS medium, autoclave, cool, and store at room temperature (23–24 °C) in the dark for 3–4 weeks. When needed, the stored hormone-free MS medium can be reheated in a microwave to melt the agar and poured into culture vessels. If hormone supplementation is required (e.g., kinetin or IBA), the appropriate stock solutions can be added to the melted medium once it cools slightly (to approximately 50 °C) before pouring. This approach saves time by eliminating the need to prepare fresh medium from scratch.
5. Seed sterilization solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Commercial bleach (4%) | 1% sodium hypochlorite | 1.25 mL |
| Tween-20 (10%) | 0.05% | 25 μL |
| Sterile Milli-Q water | 3.75 mL | |
| Total | 5 mL |
Prepare fresh. Add 1.25 mL of 4% bleach to 3.75 mL of sterile Milli-Q water. Add 25 μL of 10% Tween-20 to the bleach solution and mix well. This solution contains 1% sodium hypochlorite (from 4% commercial bleach) with 0.05% Tween-20, suitable for mild seed surface sterilization. Use for the 5-min treatment of seeds during sterilization.
Laboratory supplies
1. Sterile forceps
2. Scalpel
3. Petri dishes 94 mm × 16 mm (Sigma, catalog number: BR452000)
4. 1.5 mL sterile Eppendorf tubes (Sigma, catalog number: HS4323)
5. Sterile Whatman filter paper (90 mm diameter) (Sigma, catalog number: WHA1004090)
6. Wide-bore pipette tips (P1000); this can be prepared by cutting the ends of standard P1000 tips to create a larger orifice, then autoclaving for sterilization (cost-effective alternative)
7. Plant culture vessels (e.g., jars, tubes)
8. 50 mL Falcon tube Corning® (Sigma, catalog number: CLS430829)
9. 0.2 μm syringe filter Corning® (Sigma, catalog number: CLS431224)
Equipment
1. Laminar flow hood
2. Growth chamber or culture room with controlled temperature (27 ± 1 °C) and photoperiod (16 h light/8 h dark)
3. Light source (cool white fluorescent or LED providing 30–50 μmol·m-2·s-1)
4. Orbital shaker (for seed sterilization)
5. Autoclave
6. pH meter
Procedure
文章信息
稿件历史记录
提交日期: Jun 20, 2025
接收日期: Aug 11, 2025
在线发布日期: Aug 28, 2025
出版日期: Sep 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Deo, P. C. (2025). Practical Guide to In Vitro Clonal Propagation of Nicotiana benthamiana Using Axillary Shoot Induction. Bio-protocol 15(18): e5443. DOI: 10.21769/BioProtoc.5443.
分类
植物科学 > 植物细胞生物学 > 组织分离与培养
植物科学 > 植物育种 > 微体繁殖
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












