- EN - English
- CN - 中文
Protocol for Quantitative Estimation of Hydrogen Cyanide Production from Bacteria
细菌产氢氰酸的定量检测方案
发布: 2025年09月20日第15卷第18期 DOI: 10.21769/BioProtoc.5441 浏览次数: 1399
评审: Alba BlesaAnonymous reviewer(s)

相关实验方案

酸水解-高效液相色谱法测定集胞藻PCC 6803中聚3-羟基丁酸酯的含量
Janine Kaewbai-ngam [...] Tanakarn Monshupanee
2023年08月20日 1824 阅读

基于高效液相色谱法的史氏分枝杆菌DisA环二腺苷酸(C-di-AMP)合成酶活性研究
Avisek Mahapa [...] Dipankar Chatterji
2024年12月20日 1789 阅读
Abstract
Hydrogen cyanide (HCN) is a volatile, nitrogen-containing secondary metabolite produced by various bacterial species, primarily during the idiophase of growth under nutrient-limiting or competitive conditions. It plays a significant ecological role as a biocontrol agent by inhibiting the respiratory enzymes of plant pathogens and modulating microbial competition in the rhizosphere. Although protocols for detecting HCN production have existed for over a century, they have largely remained qualitative and are rarely optimized for quantitative assessment. This is mainly due to the volatile nature of HCN, unidentified stable reference standards, and the absence of a robust, universally accepted protocol that ensures consistency across diverse microbial types. In this study, we present a simplified and efficient colorimetric method to quantify HCN production in both Gram-positive and Gram-negative bacteria. Qualitatively, HCN production was observed by a color change due to the isopurpurate complex. This compound was then eluted and quantified by measuring absorbance at 625 nm. The method uses potassium ferrocyanide as a standard, whose slow dissociation constant enables a stable and controlled release of cyanide ions for calibration, unlike highly dissociative salts like KCN that introduce early volatilization errors. This protocol demonstrated high sensitivity, capable of detecting HCN at concentrations as low as ppm levels, with strong correlation to the standard curve (R2 > 0.99). Achieving such sensitivity with other conventional methods, such as gas detection tubes or electrochemical sensors, often requires more sophisticated instrumentation and strict handling conditions. In contrast, this approach offers a cost-effective, reproducible, and user-friendly alternative. While a universally adopted method is still lacking due to standardization challenges and HCN volatility, the proposed protocol marks a significant advancement toward accurate and accessible quantitative assessment in microbiological and agricultural applications.
Key features
• Enables both qualitative detection and quantitative estimation of hydrogen cyanide (HCN) production in bacteria using a colorimetric assay.
• Utilizes a low-dissociation reference compound, potassium ferrocyanide, to create a stable and accurate standard curve for reproducible measurement of HCN concentration.
• Offers a simple, cost-effective, and broadly applicable method suitable for screening HCN-producing bacteria in both Gram-positive and Gram-negative groups.
• Highly sensitive and accurate HCN detection at sub-ppm levels, ensuring rapid colorimetrical results and reproducibility.
Keywords: HCN (氢氰酸(HCN))Graphical overview
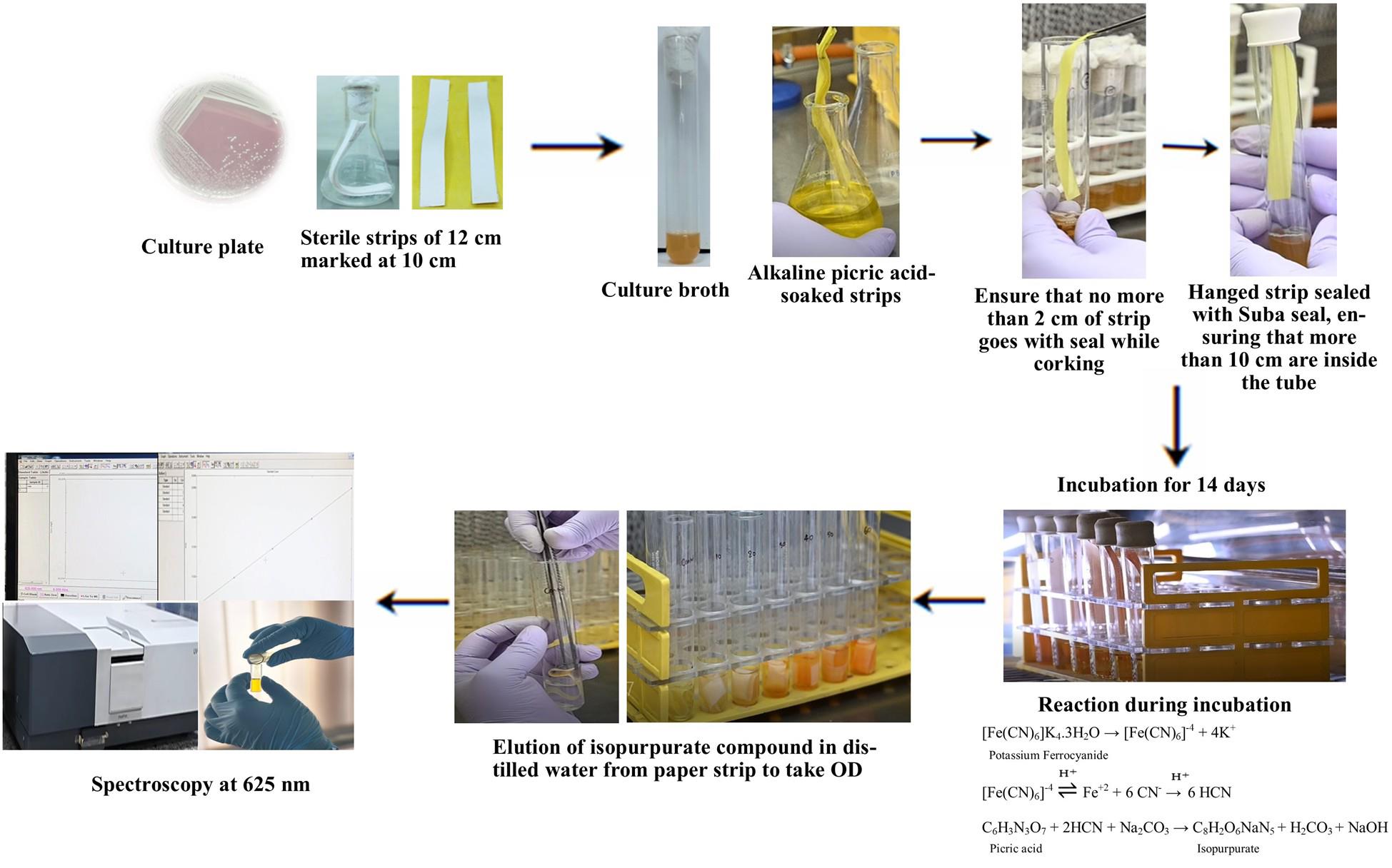
Background
Bacteria producing HCN have plant growth–promoting (PGP) characteristics, being included in biofertilizer production as a biocontrol agent [1]. The role of HCN is to display its toxic effect on the respiration of other microbes, where cyanide binds to the metal ions present in cytochrome oxidase. Particularly, it inhibits the electron transport chain by binding with the carrier ions, i.e., Cr6+and Fe3+, and checks regular energy supply, resulting in the ultimate death of the pathogens [2]. Most of the bacterial species, mainly rhizobacteria, release HCN as secondary volatile metabolites under nutrient stress conditions in their idiophase. Major HCN producers are Pseudomonas, Bacillus, Alkaligenes, and Klebsiella pneumoniae [3]. Production of the HCN is catalyzed by a membrane-bound HCN synthase complex, encoded by the hcnABC operon, which converts glycine into HCN and carbon dioxide [4]. Biosynthesis is tightly regulated and induced under specific environmental conditions such as low oxygen availability, stationary growth phase, and the presence of glycine, highlighting its role as a competitive metabolite rather than a constitutive product [5]. Regulatory systems like the anaerobic regulator ANR, the quorum-sensing transcription factors LasR/RhlR, and the GacS/GacA two-component system play central roles in controlling hcnABC expression in response to environmental and population-level cues [6,7]. These regulatory networks integrate oxygen levels, cell density, and nutrient status to fine-tune HCN production, ensuring it occurs primarily under nutrient-limiting or competitive conditions typical of the rhizosphere. Despite being toxic, HCN is efficiently secreted extracellularly, allowing the producing bacteria to inhibit surrounding pathogens while avoiding self-harm. This well-regulated secondary metabolism confers a selective advantage in microbial communities, particularly in plant-associated niches.
Some of the experimental evidence now suggests that there is no consistent correlation between the amount of HCN produced by rhizobacteria in vitro and their biocontrol performance. Specifically, strains characterized as HCN-positive (HCN) did not exhibit strong inhibition against common phytopathogens due to HCN production. In vitro assays also revealed that the minimum inhibitory concentration (MIC) of cyanide required to impact microbial growth was approximately tenfold higher than what is naturally produced by HCN-producing bacteria, suggesting that its levels in the rhizosphere are unlikely to exert broad-spectrum antimicrobial activity [8].
Instead, an alternative functional role of HCN in rhizosphere ecology is emerging, one that centers on its capacity to mobilize essential nutrients through metal chelation. Notably, HCN forms stable complexes with divalent and trivalent transition metals, such as Fe3+, Al3+, and Ca2+, thereby impacting nutrient dynamics in the soil [9]. One of the most critical implications of this interaction is its effect on phosphate (PO43-) availability. In acidic soils, phosphate readily forms insoluble complexes with iron and aluminum, limiting its accessibility to both plants and microbes [10]. In calcareous soils, similar immobilization occurs via calcium phosphate precipitation [11]. The action mechanism proposed is that HCN can bind with iron, either preventing the initial formation of insoluble Fe-PO4 complexes or releasing phosphate from already precipitated iron-phosphate compounds. The latter mechanism appears more favorable, as lower concentrations of cyanide (particularly KCN in experimental setups) were sufficient to liberate phosphate from mineral-bound forms. In controlled in vitro studies, the addition of potassium cyanide to granite-based mineral sand led to a measurable increase in both solution conductivity and phosphate concentration, confirming HCN’s role in mineral dissolution and phosphate mobilization.
Previously, qualitative estimation was based on a color change in the picric acid, which changes from a yellow color to reddish brown or dull pink. This color change occurs due to the formation of isopurpurate compound in the presence of HCN in an alkaline medium [12], which can be estimated for the quantification of the HCN produced. The ease, low cost, and flexibility of the colorimetric assay have made it a critical tool for the study of microbial HCN production.
Materials and reagents
Biological materials
1. Gram-positive isolates: Bacillus altitudinis, Bacillus amyloliquefaciens, and Bacillus haynesii
2. Gram-negative isolates: Pantoea agglomerans, Kosakonia cowanii, and Brucella intermedia
Reagents
1. Luria-Bertani (LB), Luria HiVeg broth, sterile powder (HiMedia, SKU: MV575G-500G)
2. Tryptone (HiMedia, SKU: CR014)
3. Yeast extract (HiMedia, SKU: RM027)
4. Sodium chloride (NaCl) (HiMedia, SKU: GRM031)
5. King’s B broth base, dehydrated, King’s Medium A Base (commonly called King’s B base) (HiMedia, SKU: M1543-500G)
6. Proteose peptone (HiMedia, SKU: RM005)
7. Glycerol (HiMedia, SKU: GRM081)
8. Potassium phosphate dibasic anhydrous (K2HPO4) (HiMedia, SKU: GRM3945)
9. Magnesium sulphate heptahydrate (MgSO4·7H2O) (HiMedia, SKU: PCT0008)
10. Glycine (cell culture–tested grade) (HiMedia, SKU: TC075-100G)
11. Picric acid, saturated, aqueous (HiMedia, SKU: S026-100ML)
12. Sodium carbonate anhydrous (Na2CO3) (HiMedia, SKU: MB253)
13. Potassium ferrocyanide (K4[Fe(CN)6]) (HiMedia, SKU: GRM1048)
14. Double-distilled water (ddH2O)
Solutions
1. Luria-Bertani (LB) broth (see Recipes)
2. King’s B broth (supplemented with glycine) (see Recipes)
3. Alkaline picrate solution (see Recipes)
4. Potassium ferrocyanide standard solutions (see Recipes)
Recipes
1. Luria-Bertani (LB) broth
| Reagent | Quantity or volume (per liter) |
| Tryptone | 10 g |
| Yeast extract | 5 g |
| NaCl | 10 g |
| Distilled water | up to 1 L |
Adjust pH to 7.0. Autoclave at 121 °C for 15 min.
2. King’s B broth (supplemented with glycine)
| Reagent | Quantity or volume (per liter) |
| Proteose peptone | 20 g |
| Glycerol | 10 mL |
| K2HPO4 | 1.5 g |
| MgSO4·7H2O | 1.5 g |
| Distilled water | Up to 1 L |
Adjust pH to 7.0. Autoclave at 121 °C for 15 min. Add filter-sterilized glycine to a final concentration of 4.4 g/L after autoclaving. Dispense 10 mL into each sterile test tube under aseptic conditions.
3. Alkaline picrate solution
| Reagent | Quantity or volume |
| Picric acid | 2.5 g |
| Na2CO3 | 12.5 g |
| Double-distilled water (ddH2O) | Up to 1,000 mL |
Mix both components thoroughly until fully dissolved. Store in a tightly closed amber bottle at room temperature.
4. Potassium ferrocyanide standard solutions
1. Use K4[Fe(CN)6] as a standard to prepare 0–50 ppm of CN- ion equivalents.
b. Use the following molecular conversion:
422.4 g potassium ferrocyanide = 156 g of CN-
c. Prepare standards in 10 mL of ddH2O per tube (e.g., 0, 10, 20, 30, 40, and 50 ppm CN- equivalents).
d. Cover immediately to prevent evaporation or contamination.
Laboratory supplies
Note: Prepare and autoclave sufficient double-distilled water (ddH2O) and ensure availability of sterile test tubes, conical flasks, forceps, scissors, and pipettes.
1. Sterile culture tubes or conical flasks (HiMedia, catalog numbers: PW1233 and LA449
2. Sterile measuring cylinders, conical flasks, and beakers (HiMedia, catalog numbers: LA454, LA449, and LA450
3. Sterile filter paper (Whatman No. 1 or equivalent), cut into 12 × 1 cm strips (HiMedia, catalog number: MB059)
Note: For filter paper strip preparation, first cut Whatman No. 1 or equivalent filter paper into 12 × 1 cm strips, marking at 10 cm using a pencil. Sterilize strips via autoclaving (by keeping inside the autoclavable bag). Soak sterilized strips in alkaline picrate solution for 1–2 min. Let excess drip off in sterile conditions (e.g., under a laminar flow hood). Use immediately or store in sterile, sealed containers.
4. Sterile Suba seal or rubber stopper (HiMedia, catalog number: LA410)
5. 0.22 μm syringe filter (HiMedia, catalog number: ME038)
6. Parafilm, (HiMedia, catalog number: PW009)
7. Sterile forceps and scissors (HiMedia, catalog numbers: LA396 and LA394)
8. Sterile pipettes and tips (HiMedia, catalog numbers: PW1149 and MBP001–MBP003)
Equipment
1. Spectrophotometer (SHIMADZU, model: UV-3600 UV-VIS, catalog number: UV-3600-A112950)
2. Incubator (SANCO, model: B.O.D incubator JL-1028, catalog number: SAN-200)
3. Laminar airflow cabinet (JINAN BIOBASE BIOTECH, model: BBS-DDC, catalog number: BBS11V011402003D)
4. Autoclave (iGENE LABSERVE, model: LCD Vertical Autoclave, catalog number: PUR-96-LCD)
5. Orbital shaker/incubator shaker (YIHDER, model: LM-570RD, catalog number: LM-TW/2014)
6. Vortex mixer (TARSONS, model: SPINIXTM Vortex Shaker, catalog number: 3020 SN 7903)
7. Computer (DELL, model: Intel® CoreTM i5 system)
Procedure
文章信息
稿件历史记录
提交日期: Jun 7, 2025
接收日期: Aug 7, 2025
在线发布日期: Aug 19, 2025
出版日期: Sep 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Pathak, D., Sharma, P., Govindasamy, V. and Suman, A. (2025). Protocol for Quantitative Estimation of Hydrogen Cyanide Production from Bacteria. Bio-protocol 15(18): e5441. DOI: 10.21769/BioProtoc.5441.
分类
微生物学 > 微生物新陈代谢 > 其它化合物
微生物学 > 微生物生物化学 > 其它化合物
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









