- EN - English
- CN - 中文
Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
发布: 2025年09月05日第15卷第17期 DOI: 10.21769/BioProtoc.5440 浏览次数: 3550
评审: Andrea GramaticaAnonymous reviewer(s)

相关实验方案

在平板检测仪中使用高特异性荧光探针定量巨噬细胞细胞二价铁 (Fe2+) 含量
Philipp Grubwieser [...] Christa Pfeifhofer-Obermair
2024年02月05日 2226 阅读

基于 T 细胞的平台,用于对肿瘤浸润 T 细胞的单细胞 RNA 测序数据集中鉴定的 T 细胞受体进行功能筛选
Aaron Rodriguez Ehrenfried [...] Rienk Offringa
2024年04月20日 6409 阅读
Abstract
Cardiovascular disease, the current leading cause of death worldwide, is a multifactorial disorder that involves a strong contribution of both the innate and adaptive immune systems. Overactivation of the immune system and inappropriate secretion of pro-inflammatory cytokines lead to vascular impairments and the development of cardiovascular disorders, including hypertension, atherosclerosis, and peripheral artery disease. Lymphocytes, macrophages, and dendritic cells can all secrete pro-inflammatory cytokines. This makes it challenging to isolate a specific subset of immune cells, particularly cytokines, and their contribution to vascular dysfunction remains difficult to elucidate. To solve this problem, our laboratory has developed the novel “immune cell-aorta” co-culture system described herein. This experimental protocol enables investigators to isolate an immune cell of interest and identify the cytokine(s) at the origin of vascular alterations.
Key features
• Novel ex vivo approach combining the culture of one population of immune cells with blood vessels.
• No direct contact between the cells and the blood vessels; the model enables studying the role of immune cell–derived factors or cytokines on vascular function.
• Blood vessels can subsequently be used for functional (wire/pressure myography), molecular (western blot, quantitative real-time RT-PCR), and histological studies.
Keywords: Co-culture (共培养)Graphical overview
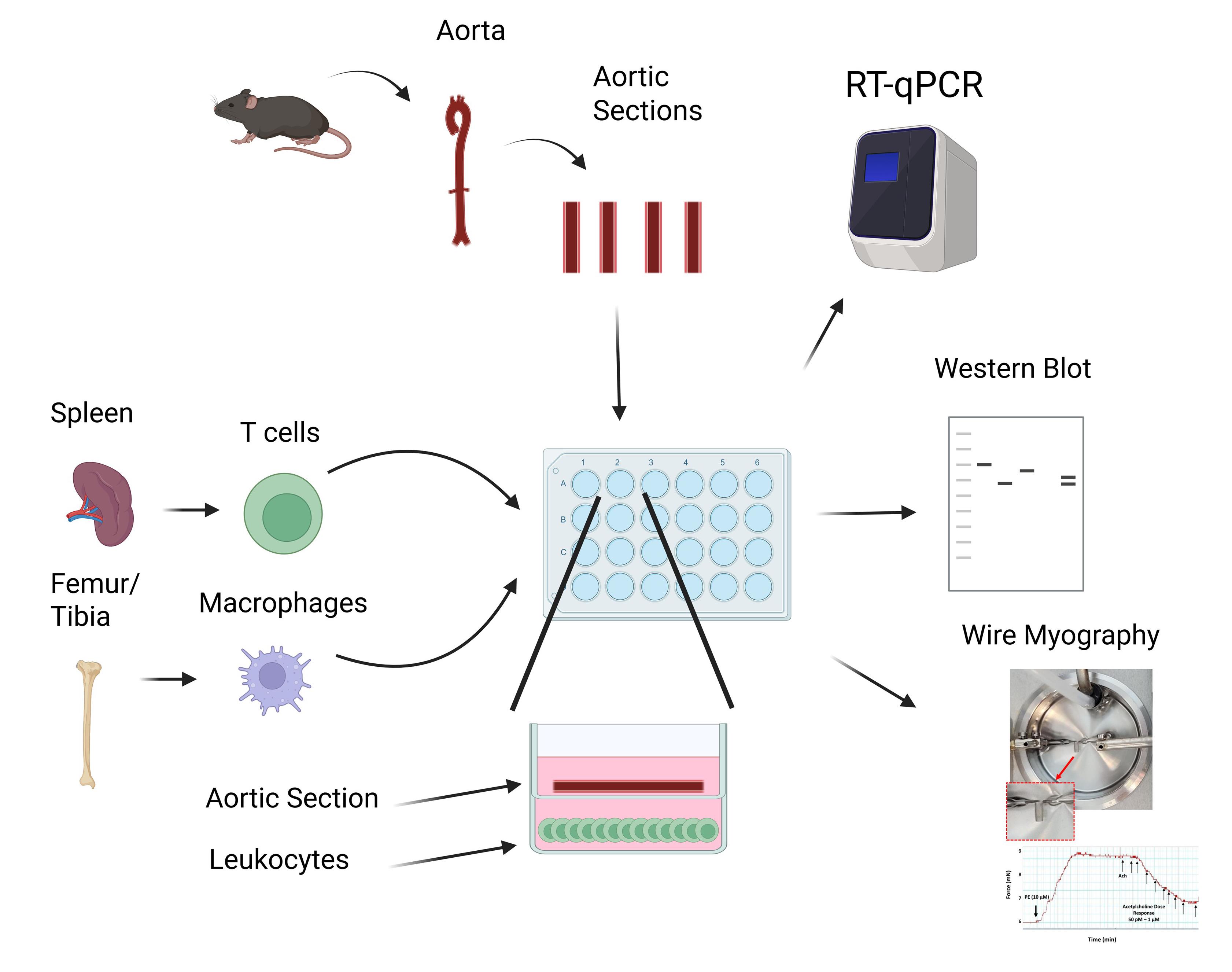 T cells and macrophages isolated from murine spleens and bone marrow, respectively, are cultured and plated in a 24-well plate. A murine aorta is excised, cleaned, sectioned, and placed into a transwell assay with 200 µL of fresh media. After overnight incubation, vessels may be extracted and cells and vessels analyzed via RT-qPCR, western blot, or wire myography.
T cells and macrophages isolated from murine spleens and bone marrow, respectively, are cultured and plated in a 24-well plate. A murine aorta is excised, cleaned, sectioned, and placed into a transwell assay with 200 µL of fresh media. After overnight incubation, vessels may be extracted and cells and vessels analyzed via RT-qPCR, western blot, or wire myography.
Background
Cardiovascular disease (CVD), including heart failure, atherosclerotic diseases, and stroke [1], is the primary cause of death worldwide and is currently on the rise [2]. Its etiopathology is complex and multifactorial, and involves, among other mechanisms, an overactivation of the immune system [3]. Hypertension, the leading modifiable cardiovascular disease risk factor, as well as atherosclerosis and other cardiovascular disorders, have all been shown to have a strong immune component and involve the contribution of both the innate [4] and adaptive immune systems [3]. Compelling evidence from both human and rodent studies highlights roles for monocytes [5], macrophages [6], dendritic cells [7], and T lymphocytes (T cells) [8]. Notably, excessive production of pro-inflammatory cytokines by the latter immune cells has been shown to cause vascular inflammation and dysfunction [9–11], both precursors of hypertension and CVD. Pro-inflammatory cytokines can act directly on blood vessels to increase their contractile phenotype [12] and vascular oxidative stress [13], and to promote endothelial nitric oxide synthase (eNOS) uncoupling [14]. However, identifying the type of immune cells producing the pro-inflammatory cytokines and the cytokine involved in the vascular alterations remains challenging; hence, the development of the protocol described here.
Macrophages can generally be split into two groups, M1 and M2 macrophages [15]. M1 macrophages, which have more of a pro-inflammatory phenotype, produce TNFα, IL-1β, IL-6, and IL-8, which contribute to endothelial dysfunction [16]. Conversely, M2 macrophages are seen as cardioprotective [17]. In our protocol, bone marrow–derived macrophages were grown and differentiated from hematopoietic stem cells using macrophage colony-stimulating factor. Macrophages can further be differentiated into the M1 subtype via the addition of lipopolysaccharide (LPS) and IFN-γ, whereas the addition of IL-4 will differentiate macrophages into the M2 subtype [18].
With respect to T cells, the two main subtypes are cytotoxic CD8+ T cells and helper CD4+ T cells [19]. The cytotoxic CD8+ T cells directly interact with and destroy infected cells via the production of pro-inflammatory cytokines such as IFN-γ and TNFα [20,21], which contribute to endothelial dysfunction via eNOS uncoupling [22] and increasing oxidative stress [23,24]. Meanwhile, CD4+ T helper cells, as opposed to CD8+ T cells, work to modulate the immune response as opposed to directly killing infected cells [25]. Naïve CD4+ T cells undergo differentiation into effector CD4+ T cells, including those with pro-inflammatory phenotypes such as Th1 [26] and Th17 [27,28], along with the anti-inflammatory T regulatory cells (Tregs) [28]. The pro-inflammatory effects of Th1 and Th17 are modulated via IFNγ, TNFα, and IL-17A [29,30]. Like IFNγ and TNFα, IL17A has also been linked to endothelial dysfunction, vascular inflammation, and hypertension [31]. Conversely, IL-10 produced by Tregs reduces inflammation and has endothelial-protective activity [32]. In addition, Tregs are increased in a female rat model of hypertension and decrease blood pressure, which demonstrates their effect on blood pressure as well as sex-specific effects [33].
Taken together, T cells and macrophages can secrete pro- and anti-inflammatory cytokines, which induce endothelial dysfunction through inflammation, downregulation, or uncoupling of eNOS, and increased oxidative stress. Our novel co-culture system provides a novel approach to investigate how cytokines produced by individual macrophage or T-cell subsets affect blood vessels, which was not previously possible. In addition to looking at vessel function, the cytokine-containing media can be analyzed to identify and quantify the cytokines present via Multiplex and ELISA. Additionally, analysis of the immune cells themselves, via flow cytometry, RT-qPCR, and western blotting, can provide more evidence of altered cytokine production.
Materials and reagents
Biological materials
1. Murine spleen collected from male and female 8–12-week-old mice for isolation of CD3+, CD4+, or CD8+ T cells
2. Murine bone marrow isolated from male and female 8–12-week-old mice for differentiation of bone marrow–derived hematopoietic stem cells into macrophages
3. Male and female murine thoracic and abdominal aortas isolated from 8–12-week-old mice for co-culture (aortas are isolated 72 h after T-cell isolation or macrophage terminal differentiation)
Reagents
1. Phosphate-buffered saline (PBS) (Fisher, catalog number: SH30256LS)
2. Bovine serum albumin (BSA) (Millipore Sigma, catalog number: 3117332001)
3. 2 mM EDTA (Millipore Sigma, catalog number: E9884-500G)
4. MACS® BSA stock solution (Miltenyi, catalog number: 130-091-376)
5. MACS® rinsing solution (Miltenyi, catalog number: 130-091-222)
6. RPMI 1640 (Fisher, catalog number: 72-400-047)
7. FBS (R&D Systems, catalog number: S11550)
8. Pen/Strep (Fisher, catalog number: SV30010)
9. TexMACS medium, 500 mL (Miltenyi, catalog number: 130-097-196)
10. Mouse IL-2 IS premium-grade TexMACS (Miltenyi, catalog number: 130-120-332)
11. Mouse M-CSF (Miltenyi, catalog number: 130-094-129)
12. 2-Mercaptoethanol (BME) (Fisher, catalog number: BP176-100)
13. Penicillin-Streptomycin (Pen/Strep) (Fisher, catalog number: SV30010)
14. Amphotericin (Fisher, catalog number: MT30003CF)
Solutions
1. T-cell isolation buffer (see Recipes)
2. T-cell culture media (see Recipes)
3. Naïve CD3+ T-cell separation media (see Recipes)
4. Macrophage differentiation/culture media (see Recipes)
Recipes
1. T-cell isolation buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sterile PBS | n/a | 20 mL |
| BSA | 0.5% | 10 mg |
| EDTA | 2 mM | 11.7 mg |
| Total | n/a | 20 mL |
Prepare a solution containing PBS, pH 7.2, 0.5% BSA, and 2 mM EDTA by diluting MACS® BSA stock solution 1:20 with autoMACS® rinsing solution.
2. T-cell culture media
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI 1640 media | n/a | 17 mL |
| FBS | 10% | 2 mL |
| Pen/Strep | 5% | 1 mL |
| Total | n/a | 20 mL |
3. Naïve CD3+ T-cell separation media
Prepare T-cell separation media by mixing 500 mL of TexMACS medium with mouse IL-2 IS premium-grade TexMACS, plus 10% FBS, 0.01 mM BME, 1:100 Pen/Strep, and 1:100 Amphotericin (add 50 U/mL of IL-2 to the media used for culture only right before use).
BME is prepared by diluting to 1:1,000. 70 μL of BME is added to each 100 mL of media.
IL-2 is prepared by dissolving it in 500 μL of PBS (stock 500 U/μL); 1 μL of diluted solution is added per 10 mL of media.
4. Macrophage differentiation/culture media
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI 1640 media | N/A | 17 mL |
| FBS | 10% | 2 mL |
| Pen/Strep | 5% | 1 mL |
| M-CSF | 20 ng/mL | 20 μL |
| Total | n/a | 20 mL |
Laboratory supplies
A. T-cell isolation
1. 1.5 mL Eppendorf tube (one per mouse) (Fisher, catalog number: 21-402-903)
2. 50 mL conical tubes (one per mouse) (Fisher, catalog number: 14-955-239)
3. 40 μM cell strainers (one per mouse) (Fisher, catalog number: 223-635-47)
4. 10 mL serological pipette (Fisher, catalog number: 12-567-603)
5. Plunger of a 1 mL syringe (one per mouse) (Fisher, catalog number: 14-817-179)
6. Pan T-Cell Isolation kit II (CD3+ T cells) (Miltenyi Biotech, catalog number: 130-095-130), CD4+ T-Cell Isolation kit (Miltenyi Biotech, catalog number: 130-104-454), CD8a+ T-Cell Isolation kit (Miltenyi Biotech, catalog number: 130-104-075)
7. LS columns (one per mouse) (Miltenyi Biotech, catalog number: 130-042-401)
8. Magnetic separator (Miltenyi, catalog number: 130-042-302; MidiMACS™ Separator on MACS® MultiStand, catalog number: 130-042-303)
9. 24-well plate with 0.4 μm polyester transwell inserts (Costar, catalog number: 3470)
B. Macrophage isolation and differentiation
1. 5 mL Eppendorf tube (one per mouse) (Fisher, catalog number: 21-402-903)
2. 100 μM cell strainers (one per mouse) (Fisher, catalog number: 22-363-549)
3. 50 mL conical tubes (one per mouse) (Fisher, catalog number: 14-955-239)
4. Plunger of a 1 mL syringe (one per mouse) (Fisher, catalog number: 14-817-179)
5. 10 mL syringe (Fisher, catalog number: 14-955-459)
6. 27-gauge ½” hypodermic needles (one per mouse) (Fisher, catalog number: 14-840-82)
7. 150 mm untreated culture plate (Thermofisher, catalog number: 150468)
Equipment
1. Dissection tools (micro scissors, scissors, forceps) (Fine Science Tools, catalog numbers: 15023-10, 14061-10, 14001-14, 11252-20, and 11274-20)
2. PipetBoy (BrandTech BRAND accu-jet pro Fisher Scientific, catalog number: 13-688-567)
3. Pasteur pipette
4. Refrigerated centrifuge for splenocytes isolation (Thermo Sorvall, model: ST16R with rotor, model: 73003181)
5. Refrigerated centrifuge for T-cell centrifugation (Labnet Prism R Refrigerated Microcentrifuge, model: C2500-R)
5. Water bath (Fisherbrand, model: Isotemp SWB15)
6. Cell culture incubator (37 °C, 5% CO2) (Heracell 150i)
7. Cell culture hood (Thermo Scientific, model: 1300 Series A2)
8. Dissection microscope (Olympus, model: SZ61)
9. Cell counter (Life Technologies Countess II)
Software and datasets
1. Prism v10 (GraphPad)
2. Microsoft Excel v16.90.2
3. BioRender (https://www.biorender.com)
Procedure
文章信息
稿件历史记录
提交日期: May 26, 2025
接收日期: Jul 30, 2025
在线发布日期: Aug 19, 2025
出版日期: Sep 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Kress, T. C., Barris, C. T., Kennard, S. and Belin de Chantemèle, E. J. (2025). Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages. Bio-protocol 15(17): e5440. DOI: 10.21769/BioProtoc.5440.
分类
免疫学 > 免疫细胞功能 > 淋巴细胞
免疫学 > 免疫细胞功能 > 巨噬细胞
医学 > 心血管疾病
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










