- EN - English
- CN - 中文
Time-Resolved cAMP Level Determination in Frog Retina Samples Using LC–MS/MS
基于LC–MS/MS的蛙视网膜样品cAMP水平的时间分辨检测
发布: 2025年09月05日第15卷第17期 DOI: 10.21769/BioProtoc.5431 浏览次数: 1249
评审: Navnita DuttaAnonymous reviewer(s)
Abstract
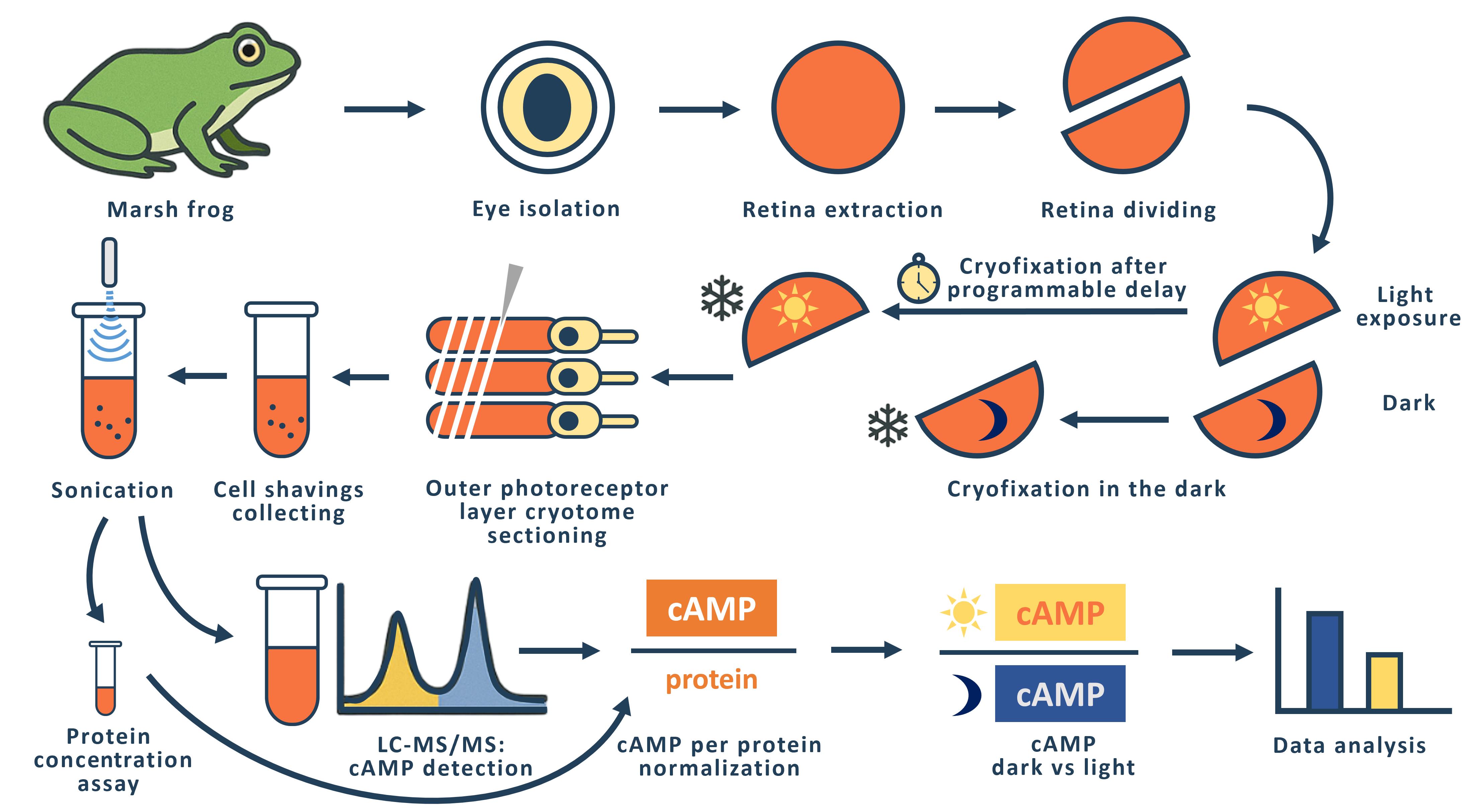
The phototransduction cascade allows photoreceptors to detect light across a wide range of intensities without saturation, with cGMP serving as the second messenger and calcium feedback as the key regulatory mechanism. While experimental evidence suggests that cAMP may also play a role in modulating this cascade, such regulation would necessitate rapid changes in cAMP levels on a timescale of seconds. However, data on the dynamics of intracellular cAMP changes in photoreceptors remain scarce, primarily due to the limitations of conventional fluorescence-based methods in this specialized sensory system. To address this gap, we developed a methodology combining rapid cryofixation of retinal samples following light stimulation with the isolation of outer segment preparations. The rapid cryofixation setup comprises six computer-controlled sections, each with a high-speed stepper motor-driven lever that rapidly moves the specimen in a 180° arc within ~80 ms to press it against a liquid nitrogen-cooled copper cylinder for fixation. Using highly sensitive metabolomics techniques, we measured cAMP levels in these samples. This approach enables the investigation of rapid cAMP dynamics and its potential regulatory role in phototransduction, providing a foundation for understanding the interplay between cAMP and PKA signaling in photoreceptor function.
Key features
• The protocol provides ms time resolution in retina outer segment sampling in response to light stimulus with cryofixation, conserving proteome and metabolome response features.
• The protocol allows direct cAMP quantification with an average level of 11.4 ± 0.5 pmol/mg of protein in the dark.
Keywords: Retina (视网膜)Graphical overview
Background
In the realm of vertebrate visual systems, the phototransduction cascade plays a crucial role in enabling effective vision across a vast spectrum of light intensities, from the dimmest of night conditions to the brightness of midday. This intricate biochemical pathway operates through a multi-stage amplification system, characterized by several feedback loops, allowing photoreceptors to adapt to varying illumination levels. The secondary messengers involved in this process, cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP), each serve distinct yet interrelated functions within the cascade. While the role of cGMP in regulating the permeability of photoreceptor plasma membrane channels has been extensively documented [1,2], the nuanced roles of cAMP have garnered less attention, despite its significant involvement in various photoreceptor processes, including modulation of circadian rhythms and the dynamics of retinomotor movements [3–8].
Previous studies have demonstrated that several key proteins within the phototransduction pathway—such as phosphodiesterase type 6 (PDE6) and rhodopsin kinase—are susceptible to phosphorylation by protein kinase A (PKA), suggesting a regulatory interplay between cAMP levels and the efficiency of the phototransduction cascade [8,9]. However, the dynamics of cAMP concentration within photoreceptors remain inadequately characterized, particularly concerning the rapid fluctuations necessary for real-time regulation of the cascade's activity.
Existing protocols for measuring intracellular signaling, including cAMP dynamics, have predominantly relied on fluorescent methods, which are often unsuitable for the unique conditions pertinent to photoreceptor physiology. The potent light emission employed in the fluorescence technique itself constitutes an adequate stimulus for the phototransduction cascade. The rapid cryofixation technique utilized in this method presents significant advantages over these conventional approaches, allowing for precise measurement of cAMP levels immediately following light stimulation. By employing highly sensitive metabolomics techniques paired with cryofixed samples, we provide a robust protocol that significantly enhances our ability to capture real-time biochemical changes during phototransduction.
The general idea of rapid freezing of retinal fragments was previously proposed and realized by Govardovskii [10] and Blazynski and Cohen [11, 12], who used this approach to study the effect of light stimulation on intracellular cGMP levels in amphibian outer segments. In both cases, the cryofixation surface was a mirror-polished surface of a copper cylinder cooled to the temperature of liquid nitrogen [10] or liquid helium [11]. The Govardovskii setup allowed simultaneous fixation of four retinal fragments with a minimum sample cryofixation time of 500 ms. We refer to the minimum time here as the time interval from command to cryofixation of the specimen. The Blazinski setup was a single-channel unit with a minimum cryofixation time of 47 ms.
In designing our setup, we considered that it should be multi-channel and allow us to fix retinal fragments from two eyes of an animal in one experiment. Experimentally, we have found that the optimal size of a retinal fragment for obtaining a tissue sample using a cryotome is 1/2 to 1/3 of the retina of one eye. Thus, in one experiment, it is possible to simultaneously fixate and then measure cAMP levels in 4–6 retinal fragments from two eyes of an animal. To perform such an experiment, we created a setup consisting of six identical sections in which a fast-moving stepper motor (maximum speed 150,000 rpm) drives a lever with a retinal sample pad. The retinal sample, placed on a small circle of filter paper, is placed horizontally on the pad and is illuminated with LED light (λmax= 525 nm) on command from the computer. The intensity of the light used to stimulate the retina can be varied within a range of 3.6 × 103–3.6 × 105 quanta/(s·μm2). If necessary, a grey light filter can be used to further attenuate the light flux. Synchronous illumination of all six retinal specimens is achieved by a special design of the light guide that carries the light from the LED to the retinal specimens. The light guide has six identical fiber bundles at one end, each of which illuminates one location with a retinal fragment. At the other end, the fibers from all six terminations are combined into a single bundle by uniform and random mixing. The end of this bundle is illuminated through a condenser by an LED.
After a programmable delay, the sample arm arcs 180° and presses the retinal sample against the polished surface of a copper cylinder cooled to liquid nitrogen temperature. The time taken for the lever to move from the command stage to making contact with the surface of the copper cylinder (the minimum sample cryofixation time) was measured using a slow-motion video of the lever's rotation. The results of multiple measurements showed a small variation in the individual minimum cryofixation times, which for channels 1–6 are 153 ± 12, 94 ± 9, 92 ± 3, 97 ± 6, 90 ± 1, and 110 ± 2 (shown as average ± SD, ms, n = 3–5). The average time for all channels is 106 ms. We used the measured minimum cryofixation times to calculate the actual cryofixation delay time for each individual channel. To prevent the retinal specimens from flying off the platform during the rapid movement of the lever, we glued filter paper circles on which the retinal specimens were placed to the platform on the lever using Tissue-Tek gel. To enable the Tissue-Tek frozen after cryofixation to be easily removed from the lever platform, we made a composite platform with a fluoroplastic core.
Materials and reagents
Biological materials
Adult marsh frogs (Pelophylax ridibundus) were collected from the wild in southern Russia. Frogs were housed in water tanks at 6–8 °C for a maximum of 8 months. Animals were handled in accordance with the Council Directive of the European Communities (24 November 1986; 86/609/EEC), and the experimental protocol was approved by the local Institutional Animal Care and Use Committee (protocol # 4/22 at 28.01.2022).
Reagents
1. Liquid nitrogen
2. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653)
3. Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333)
4. Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M1028)
5. Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: 21115)
6. Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761)
7. HEPES (Sigma-Aldrich, catalog number: H3375)
8. Glucose (D-Glucose) (Sigma-Aldrich, catalog number: G8270)
9. Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E5134)
10. Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 72068)
11. Tissue-Tek (Sakura, catalog number: 4583)
12. Coomassie Brilliant Blue G-250 (Helicon, catalog number: SRL-64222-5G)
13. Ethanol (ethyl alcohol, 96%) (LenReaktiv, catalog number: 000164)
14. Phosphoric acid (H3PO4) (Sigma-Aldrich, catalog number: 345245)
15. Bovine serum albumin (BSA) (Dia-M, catalog number: BSA.0100)
16. Cyclic adenosine monophosphate (cAMP) (Merck, catalog number: A9501)
17. Сyclic guanosine monophosphate (cGMP) (Merck, catalog number: G6129)
18. Acetonitrile (Cryochrom, Russia)
19. Hydrochloric acid (Supelco, catalog number:109057)
20. Ammonium formate (CDH, catalog number: 027169)
21. Isotopic-labeled cyclic adenosine monophosphate (cAMP-13C5) (TRC, Canada, catalog number: A280457)
Solutions
1. Frog Ringer (FR) (see Recipes)
2. Bradford reagent (see Recipes)
3. Mass-spec solution (see Recipes)
Recipes
1. Frog Ringer (FR)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 90 mM | 18 mL, 5 M |
| KCl | 2.5 mM | 2.5 mL, 1 M |
| MgCl2 | 1.4 mM | 1.4 mL, 1 M |
| CaCl2 | 1.05 mM | 1.05 mL, 1 M |
| NaHCO3 | 5 mM | 5 mL, 1 M |
| HEPES | 5 mM | 5 mL, 1 M |
| Glucose | 10 mM | 10 mL, 1 M |
| EDTA | 0.05 mM | 0.1 mL, 0.5 M |
| NaOH | To pH 7.6 | |
| MQ water | To 1,000 mL |
2. Bradford reagent
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol | 5% | 50 mL |
| Coomassie G-250 | 100 mg/L | 100 mg |
| H3PO4 | 10% | 100 mL |
| MQ water | To 1,000 mL |
3. Mass-spec solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ammonium formate | 0.1 M | 6.30 g |
| MQ water | To 1,000 mL |
Laboratory supplies
1. Microcentrifuge tubes, 0.5 and 1.5 mL (SSIbio, catalog numbers: 1110-00 and 1210-00)
2. Filter paper (ECOS-1, catalog number: A200042894902)
3. 96-well (Medpolymer, catalog number: 112202) and 6-well plates (NEST, catalog number: 703011)
4. 3 cm (Jet Biofil, catalog number: TCD000035) and 10 cm Petri dishes (Medpolymer, catalog number: 124124)
5. Hollow metal tube: custom-made copper tube with an outer diameter of 8 mm and a wall thickness of 0.7 mm
6. Metal spatulas
7. Dissecting scalpels and blades, dissecting scissors, dissecting dressing tweezers
8. Light-isolating cryo-container: custom-made, constructed from a metal box filled with liquid nitrogen, featuring metal cylinders that support the sample platform above; the exterior of the cryocontainer is insulated with foam to minimize thermal exchange
Equipment
1. Centrifuge for Eppendorf tubes (Eppendorf miniSpin plus, Eppendorf, Germany)
2. Sonicator (Elmasonic S30, Elma-Hans Schmidbauer GmbH, Germany)
3. Vortex MultiReax (Heidolph instruments GmbH, Germany)
4. Chromatography column (Agilent Zorbax SB-C8 150 mm × 4.6 mm × 1.8 μm, Agilent Technologies, USA)
5. High-performance liquid chromatograph Dionex UltiMate 3000 with QExactive high-resolution mass-spectrometry detector with electrospray ionization (Thermo Fisher Scientific)
6. AUW-220D analytic balance (Shimadzu, Japan)
7. Hole punch with hole diameter of 1 cm
8. Red light/dark room
9. Infrared observation system (custom-made)
10. Milli-Q® water system (Merck Life Science, model: Ultrapure, Type 1)
11. Stereomicroscope (Nexcope, model: NSZ-810)
12. Cryotome (Leica, rotary freezing cryotome, model: RM2265)
13. Sonicator (Sonics, model: Vibra-Cell, VCX130)
14. Drybath (Hangzhou Allsheng Instruments, model: MK-20)
15. CLARIOstar Plus microplate reader (Labtech International)
16. Cryofixation device (custom-made)
17. Lyophilizer (Labconco, catalog number: 7310031)
Software and datasets
1. ThermoXcalibur Version 4.1.31.9 (Thermo Fisher Scientific)
2. Microsoft Excel 2010 (Microsoft)
Procedure
文章信息
稿件历史记录
提交日期: Apr 12, 2025
接收日期: Jun 27, 2025
在线发布日期: Aug 8, 2025
出版日期: Sep 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Chernyshkova, O. V., Belyakov, M. V., Meshalkina, D. A. and Firsov, M. L. (2025). Time-Resolved cAMP Level Determination in Frog Retina Samples Using LC–MS/MS. Bio-protocol 15(17): e5431. DOI: 10.21769/BioProtoc.5431.
分类
神经科学 > 感觉和运动系统 > 视网膜
生物化学 > 其它化合物
系统生物学 > 代谢组学 > 神经代谢物
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link