- EN - English
- CN - 中文
Simultaneous Capture of Chromatin-Associated RNA and Global RNA–RNA Interactions With Reduced Input Requirements
低样本量条件下同时捕获染色质相关RNA及全局RNA–RNA互作
(*contributed equally to this work) 发布: 2025年09月05日第15卷第17期 DOI: 10.21769/BioProtoc.5430 浏览次数: 3600
评审: Wei DaiAnonymous reviewer(s)
Abstract
Chromatin-associated RNAs (caRNAs) have been increasingly recognized as key regulators of gene expression and genome architecture. A few technologies, such as ChRD-PET and RedChIP, have emerged to assess protein-mediated RNA–chromatin interactions, but each has limitations. Here, we describe the TaDRIM-seq (targeted DNA-associated RNA and RNA–RNA interaction mapping by sequencing) technique, which combines Protein G (PG)-Tn5-targeted DNA tagmentation with in situ proximity ligation to simultaneously profile caRNAs across genomic regions and capture global RNA–RNA interactions within intact nuclei. This approach reduces the required cell input, shortens the experimental duration compared to existing protocols, and is applicable to both mammalian and plant systems.
Key features
• A multi-omics sequencing strategy.
• Compatible with mammalian and plant systems.
• Profiling of epigenome, RNA–DNA, and RNA–RNA interactomes.
Keywords: Chromatin-associated RNAs (caRNAs) (染色质相关RNA(caRNA))Graphical overview
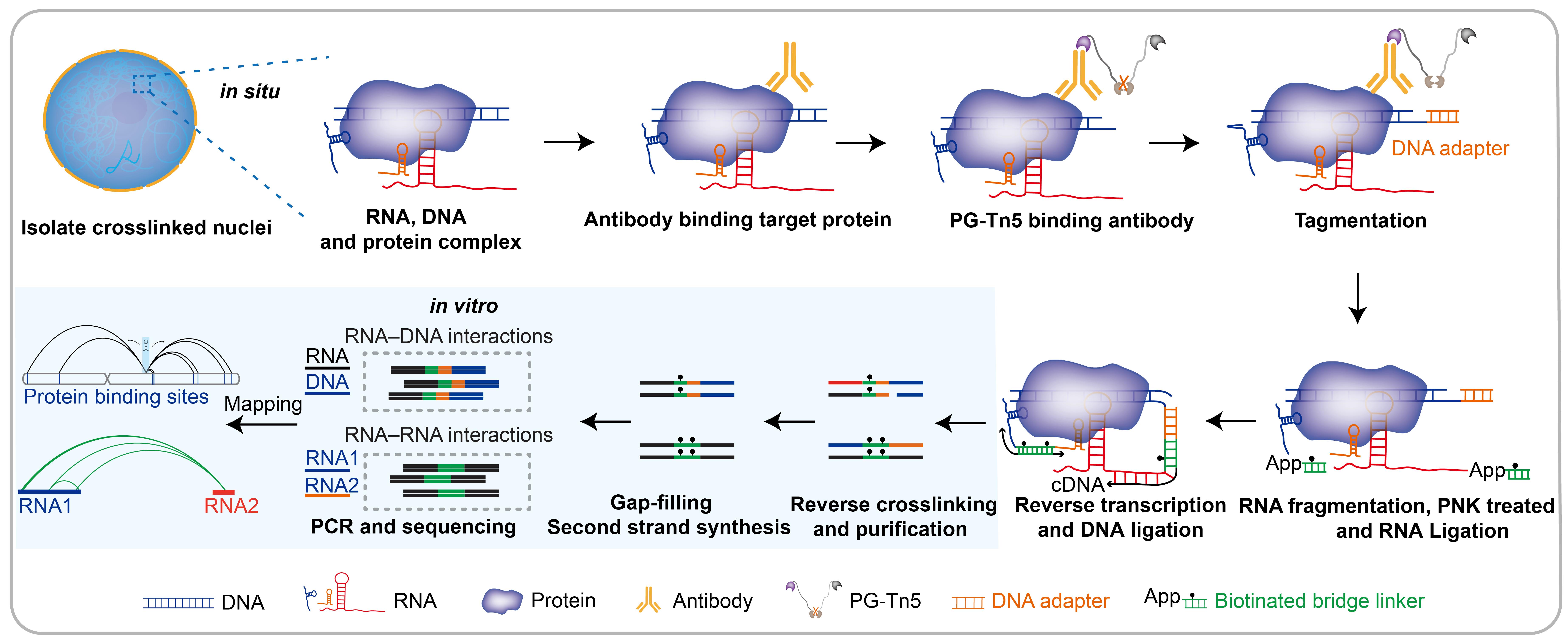
Targeted DNA-associated RNA and RNA–RNA interaction mapping by sequencing (TaDRIM-seq) technique schematic
Background
Chromatin-associated RNAs (caRNAs) have been proposed to represent an additional regulatory layer of the epigenome [1]. Some caRNAs act in trans, being recruited to distant genomic loci (trans-acting RNAs), while others function in cis, remaining enriched at their transcription sites (cis-acting RNAs) [2]. Increasing evidence has demonstrated that caRNAs play crucial roles in regulating gene transcription [3–5], X-chromosome inactivation [6], and the three-dimensional organization of the genome [7,8].
Current methods, such as ChRD-PET [9] and RedChIP [10], enable the identification of DNA elements targeted by specific caRNAs. However, these approaches are resource-intensive, time-consuming, and inefficient due to in vitro ligation limitations, and they are unable to capture RNA–RNA interactions. To overcome these limitations, we recently developed a method named TaDRIM-seq [11]. In this protocol, we provide a step-by-step guide for performing TaDRIM-seq in rice and human cells. The assay is also compatible with other cell lines, as well as plant and animal tissues, making it broadly applicable to the study of caRNAs-mediated chromatin regulation.
Materials and reagents
Biological materials
1. Human K562 (ATCC, catalog number: CCL-243)
2. Rice leaf tissue [Xian/indica cv. Minghui 63 (MH63)]
Reagents
1. Formaldehyde (37%) (Sigma-Aldrich, catalog number: F8775)
2. Glycine (Sigma-Aldrich, catalog number: G7126-1kg)
3. PBS (10×) (Invitrogen, catalog number: AM9625)
4. HEPES (1 M) (Coolaber, catalog number: SL6056-500mL)
5. NaCl (5 M) (Invitrogen, catalog number: AM9759)
6. Spermidine (Sigma-Aldrich, catalog number: S2626-1g)
7. Protease inhibitor cocktail tablets (Roche, catalog number: 58914000)
8. RiboLock RNase inhibitor (40 U/μL) (Invitrogen, catalog number: EO0381)
9. Nuclease-free water (NF-H2O) (Thermo Fisher Scientific, catalog number: EF0651)
10. Digitonin (5%) (Thermo Fisher Scientific, catalog number: BN2006)
11. EDTA (0.5 M) (Invitrogen, catalog number: AM9260G)
12. BSA (20 mg/mL) (NEB, catalog number: B9000S)
13. MgCl2 (1 M) (Invitrogen, catalog number: AM9530G)
14. SDS (10%) (Invitrogen, catalog number: AM9822)
15. dNTPs (10 mM) (Invitrogen, catalog number: 18427088)
16. Triton X-100 (Sigma-Aldrich, catalog number: 9036-19-5)
17. H3K4me3 antibody (50 μg) (Abcam, catalog number: ab8580)
18. H3K27me3 antibody (100 μg) (Abcam, catalog number: ab6002)
19. Tris-HCl (1 M, pH 7.5) (Invitrogen, catalog number: 15567027)
20. rCutSmartTM buffer (10×) (NEB, catalog number: B6004V)
21. T4 polynucleotide kinase (T4 PNK, 10 U/μL) (Thermo Fisher Scientific, catalog number: EK0032)
22. T4 polynucleotide kinase reaction buffer (10×) (Thermo Fisher Scientific, catalog number: EK0032)
23. 5′ DNA Adenylation kit (NEB, catalog number: E2610L)
24. T4 RNA ligase 2, truncated KQ (200 U/μL) (NEB, catalog number: M0373L)
25. T4 RNA ligase reaction buffer (10×) (NEB, catalog number: B0216S)
26. PEG 8000, 50% (w/v) (NEB, catalog number: B1004S)
27. T4 DNA Ligase (400 U/μL) (NEB, catalog number: M0202S)
28. DNA ligase reaction buffer (10×) (NEB, catalog number: B0202S)
29. Phenol/chloroform/isoamyl alcohol (phenol/ChCl3/IAA, 25:24:1, pH 7.9) (Ambion, catalog number: AM9730)
30. Sodium acetate (3 M, pH 5.2) (VWR, catalog number: E521-100mL)
31. Ethanol absolute (pure) (Sangon Biotech, catalog number: A500737)
32. T4 DNA polymerase (3 U/μL) (NEB, catalog number: M0203L)
33. NEBufferTM r2.1 (10×) (NEB, catalog number: B6002S)
34. Tween 20 (Sigma-Aldrich, catalog number: P9416-100ml)
35. Isopropanol (Sigma-Aldrich, catalog number: I-9516-500ml)
36. Buffer EB (QIAGEN, catalog number: 19086-250ml)
37. ATP (10 mM) (NEB, catalog number: P0756L)
38. RNase remover and nucleic acid decontamination reagent (Vazyme, catalog number: R504)
39. Hyperactive pG-Tn5 Transposase (500 ng/μL) (Vazyme, catalog number: S602)
40. TruePrep DNA Library Prep Kit V2 for Illumina (Vazyme, catalog number: TD501)
41. DNA Clean Beads (Vazyme, catalog number: N411-03)
42. DynabeadsTM M-280 (10 mg/mL) (Invitrogen, catalog number: 11205D)
43. I-Block protein-based blocking reagent (Thermo Fisher, catalog number: T2015)
44. M-MuLV reverse transcriptase (200 U/μL) (NEB, catalog number: M0253L)
45. M-MuLV reverse transcriptase reaction buffer (10×) (NEB, catalog number: B0253L)
46. RNase IF (NEB, catalog number: M0243S)
47. NEBNext® second strand synthesis enzyme mix (NEB, catalog number: E6111L)
48. DNA Clean & Concentrator-5 (Zymo, catalog number: D4013)
49. Oligo Clean & Concentrator (Zymo, catalog number: D4060)
50. Primers (synthesized by Sangon Biotech with HPLC purification)
Solutions
1. Buffer S (see Recipes)
2. Buffer F (see Recipes)
3. 1× wash buffer A (see Recipes)
4. Antibody buffer (see Recipes)
5. 1× transposase incubation buffer (see Recipes)
6. 1× wash buffer B (see Recipes)
7. Lysis buffer (see Recipes)
8. Proteinase K solution buffer (see Recipes)
9. iBlock buffer (see Recipes)
10. 2× binding and washing buffer (see Recipes)
11. 1× binding and washing buffer (see Recipes)
12. 2× SSC/0.5% (w/v) SDS (see Recipes)
13. Sheared DNA solution (see Recipes)
Recipes
1. Buffer S
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M HEPES | 50 mM | 0.5 mL |
| 5 M NaCl | 150 mM | 0.3 mL |
| 0.5M EDTA | 1 mM | 0.02 mL |
| Triton X-100 | 1% | 0.1 mL |
| 10% sodium deoxycholate | 0.1% | 0.1 mL |
| 10% SDS | 1% | 1 mL |
| 50× protease inhibitors | 1× | 0.2 mL |
| RiboLock RNase (40 U/μL) | 0.2 U/μL | 0.05 mL |
| H2O | n/a | 7.73 mL |
| Total | n/a | 10 mL |
Note: This buffer should be prepared fresh before use.
2. Buffer F
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M HEPES | 50 mM | 0.5 mL |
| 5 M NaCl | 150 mM | 0.3 mL |
| 2 M Spermidine | 1 mM | 0.005 mL |
| Triton X-100 | 1% | 0.1 mL |
| 10% sodium deoxycholate | 0.1% | 0.1 mL |
| 50× protease inhibitors | 1× | 0.2 mL |
| RiboLock RNase (40 U/μL) | 0.2 U/μL | 0.05 mL |
| H2O | n/a | 8.745 mL |
| Total | n/a | 10 mL |
Note: This buffer should be prepared fresh before use.
3. 1× wash buffer A
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M HEPES (pH 7.6) | 20 mM | 100 μL |
| 5 M NaCl | 150 mM | 150 μL |
| 2 M Spermidine | 0.5 mM | 1.25 μL |
| 50× protease inhibitors | 1× | 100 μL |
| RiboLock RNase (40 U/μL) | 0.2 U/μL | 25 μL |
| H2O | n/a | 4.623 mL |
| Total | n/a | 5 mL |
Note: This buffer should be prepared fresh before use.
4. Antibody buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1× wash buffer A | n/a | 495 μL |
| 0.5M EDTA | 2 mM | 2 μL |
| 30% BSA | 0.1% | 1.6 μL |
| 5% Digitonin | 0.05% | 5 μL |
| Total | n/a | 503.6 μL |
Note: This buffer should be prepared fresh before use.
5. 1× transposase incubation buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M HEPES (pH 7.6) | 20 mM | 100 μL |
| 5 M NaCl | 300 mM | 300 μL |
| 2 M Spermidine | 0.5 mM | 1.25 μL |
| 5% Digitonin | 0.01% | 10 μL |
| 50× protease inhibitors | 1× | 100 μL |
| RiboLock RNase (40 U/μL) | 0.2 U/μL | 25 μL |
| H2O | n/a | 4.467 mL |
| Total | n/a | 5 mL |
Note: This buffer should be prepared fresh before use.
6. 1× wash buffer B
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl (pH 7.5) | 20 mM | 1 mL |
| 1 M MgCl2 | 10 mM | 0.5 mL |
| 10% Tween 20 | 0.2% | 1 mL |
| RiboLock RNase (40 U/μL) | 0.2 U/μL | 0.25 mL |
| H2O | n/a | 47.25 mL |
| Total | n/a | 50 mL |
Note: This buffer should be prepared fresh before use.
7. Lysis buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl (pH 7.5) | 10 mM | 10 μL |
| 5 M NaCl | 10 mM | 2 μL |
| 10% NP40 | 0.2% | 20 μL |
| 50× protease inhibitors | 1× | 20 μL |
| RiboLock RNase (40 U/μL) | 0.2 U/μL | 5 μL |
| H2O | n/a | 943 μL |
| Total | n/a | 1 mL |
Note: This buffer should be prepared fresh before use.
8. Proteinase K solution buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl (pH 7.5) | 50 mM | 50 μL |
| 10% SDS | 1% | 100 μL |
| 5 M NaCl | 100 mM | 20 μL |
| 0.5 M EDTA | 1 mM | 2 μL |
| Proteinase K (20mg/mL) | 1 mg/mL | 50 μL |
| H2O | n/a | 778 μL |
| Total | n/a | 1 mL |
Note: This buffer should be prepared fresh before use.
9. iBlock buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| iBlock | 2% (w/v) | 0.2 g |
| 10% SDS | 0.5% | 0.5 mL |
| H2O | n/a | 9.5 mL |
| Total | n/a | 10 mL |
Note: Incubate the reagent in a 65 °C water bath to facilitate dissolution. The buffer is stable for up to 6 months when stored at room temperature.
10. 2× binding and washing buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl (pH 8) | 10 mM | 0.5 mL |
| 5 M NaCl | 2 M | 20 mL |
| 0.5 M EDTA | 1 mM | 0.1 mL |
| H2O | n/a | 29.4 mL |
| Total | n/a | 50 mL |
Note: This buffer is stable for up to 6 months when stored at 4 °C.
11. 1× binding and washing buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl (pH 8) | 10 mM | 0.5 mL |
| 5 M NaCl | 1 M | 10 mL |
| 0.5 M EDTA | 1 mM | 0.1 mL |
| H2O | n/a | 39.4 mL |
| Total | n/a | 50 mL |
Note: This buffer is stable for up to 6 months when stored at 4 °C.
12. 2× SSC/0.5% (w/v) SDS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 20× SSC | 2× | 5 mL |
| 10% SDS | 0.5% | 2.5 mL |
| H2O | n/a | 42.5 mL |
| Total | n/a | 50 mL |
Note: This buffer can be stored at room temperature for up to 3 months; however, lower room temperatures may cause SDS to precipitate. If this occurs, place the buffer in a 25 °C water bath to fully dissolve the SDS before use.
13. Sheared DNA solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sheared DNA | 0.5 ng/μL | x μL |
| 2× binding and washing buffer | 1× | 50 μL |
| H2O | n/a | 50 – x μL |
| Total | n/a | 100 μL |
Note: The genomic DNA isolated from plant or animal was fragmented into 300–500 bp fragments by sonication using a Bioruptor (Diagenode) set to high intensity with 30 cycles (30 s ON, 50 s OFF) at 4 °C. The sheared DNA solution is stable for up to 6 months when stored at -20 °C.
Equipment
1. Eppendorf Thermo Mixer® C (Eppendorf, model: ThermoMixer C)
2. Centrifuge (Eppendorf, model: 5425R)
3. DynaMag-2 Magnet (Life Technologies, catalog number: 12321D)
4. Thermal Cycler (Bio-Rad, model: T100TM)
5. Vortex-Genie Mixers (VWR, catalog number: 58816-121)
6. Low-temperature incubator (Being, catalog number: BC-60L)
7. Qubit 2.0 Fluorometer (Invitrogen, catalog number: Q32866)
8. Intelli-mixer RM-2L (Rose Scientific, catalog number: MX1000)
9. Bioruptor® Plus sonication device (Diagenode)
Software and datasets
1. All data and code have been deposited to GitHub: https://github.com/304243504/HiR
Procedure
文章信息
稿件历史记录
提交日期: May 28, 2025
接收日期: Jul 22, 2025
在线发布日期: Aug 11, 2025
出版日期: Sep 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Ding, C., Chen, G., Luan, S., Gong, Y., Gui, C., Yang, C., Xiang, Z., Du, J., Foda, M. F., Yan, J. and Li, X. (2025). Simultaneous Capture of Chromatin-Associated RNA and Global RNA–RNA Interactions With Reduced Input Requirements. Bio-protocol 15(17): e5430. DOI: 10.21769/BioProtoc.5430.
分类
生物信息学与计算生物学
分子生物学 > RNA > miRNA与mRNA 的相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












