- EN - English
- CN - 中文
Assessing Human Treg Suppression at Single-Cell Resolution Using Mass Cytometry
利用质谱流式在单细胞分辨率下评估人Treg的抑制作用
发布: 2025年08月20日第15卷第16期 DOI: 10.21769/BioProtoc.5424 浏览次数: 2876
评审: Alka MehraAnonymous reviewer(s)
Abstract
Regulatory T cells (Tregs) are essential for maintaining immune balance by controlling the activation and expansion of other immune cells. Conventional suppression assays often rely on co-culturing purified cell populations, which limits multiplexed phenotyping and physiological relevance. This protocol describes a high-dimensional, single-cell assay for profiling Treg-mediated suppression within a peripheral blood mononuclear cell (PBMC) system. Tregs are first isolated by cell sorting and then reintroduced into autologous PBMCs at defined ratios. A 52-marker mass cytometry (CyTOF) panel is used to quantify cell division and phenotypic responses across multiple immune subsets. This approach allows for integrated analysis of Treg function with broad compatibility for patient profiling and drug evaluation.
Key features
• Quantifies Treg-mediated suppression in autologous PBMCs at single-cell resolution.
• Enables high-dimensional phenotyping and proliferation tracking across multiple immune subsets using a 52-marker CyTOF panel.
• Maintains physiological relevance by assessing suppression in a complex PBMC environment.
• Compatible with patient-derived samples and drug perturbation experiments for translational immunology applications
Keywords: Regulatory T cells (调节性T细胞)Graphical overview

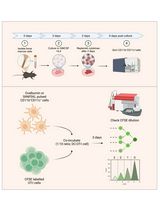
Single-cell suppression profiling of human Tregs. On the first day of the procedure, peripheral blood mononuclear cells (PBMCs) are thawed and CFSE-stained. Subsequently, Tregs are isolated by magnetic- and flow-assisted cell sorting. The Treg-negative PBMCs and the Tregs are mixed together in desired ratios and stimulated for 5 days. On day 5, cells are harvested and stained for mass cytometry (CyTOF). On day 6, data is acquired, normalized, and exported. On day 7 to approximately day 14, data is analyzed mainly in R.
Background
Regulatory T cells (Tregs) are a specialized subset of CD4+ T cells that play a central role in immune tolerance by suppressing activation and expansion of conventional T cells and other immune populations. Treg dysfunction has been implicated in a wide range of diseases, including autoimmunity, chronic inflammation, cancer, and infectious diseases [1,2]. To study Treg activity in vitro, suppression assays have traditionally relied on co-cultures of sorted Tregs and responder T cells, typically CD4+CD25- or CD8+ T cells, in the presence of polyclonal stimulation [3–5]. Suppressive function is commonly read out by measuring proliferation, cytokine production, or activation marker expression in the responder cells [3–5]. While these assays have been foundational for dissecting Treg biology, they are limited by their reductionist nature and the need for purified cell populations [5–9].
Standard suppression assays are often conducted with only one or two responder cell types, thereby excluding interactions with other immune populations that are part of the physiological context in vivo. Furthermore, they offer limited resolution for multiplexed phenotyping and functional tracking of individual cells. More recently, high-dimensional single-cell technologies such as mass cytometry, also known as cytometry by time of flight (CyTOF), have enabled simultaneous measurement of dozens of parameters across diverse immune subsets. CyTOF is a single-cell analysis technology that combines flow cytometry with mass spectrometry. In CyTOF, antibodies are tagged with metal isotopes rather than fluorophores, enabling the simultaneous quantification of over 50 parameters without spectral overlap. Cells are ionized, and the attached metal tags are detected by time-of-flight mass spectrometry, allowing precise quantification of marker expression [10–12]. This technology has opened new avenues for functional immune profiling [13–18]. However, protocols leveraging this technology to assess Treg function in a more holistic, system-level context have remained underdeveloped until recently [19].
The protocol presented here, referred to as single-cell suppression profiling of human Tregs (scSPOT), integrates reconstitution of sorted Tregs into autologous peripheral blood mononuclear cells (PBMCs) at controlled ratios, followed by in vitro stimulation and mass cytometry–based analysis [19]. Using the recommended 52-marker CyTOF panel, this approach allows simultaneous assessment of Treg suppressive effects on multiple immune cell types, including CD4+ and CD8+ T cells, B cells, NK cells, and myeloid subsets. Importantly, it captures both proliferative responses and phenotypic shifts within a complex immune environment, without the need for prior responder cell purification. The protocol is well-suited for evaluating inter-individual variation, analyzing patient samples, or screening drug effects on immune regulation.
Compared to conventional suppression assays, scSPOT offers improved physiological relevance by preserving the cellular diversity of PBMCs and provides high-resolution, multidimensional data at the single-cell level. While the approach requires access to mass cytometry infrastructure, it enables a deeper and broader view of Treg function and its impact on the immune landscape. As such, it represents a valuable tool for both basic research and translational immunology applications, especially in contexts where immune modulation is of therapeutic interest. Although we provide a detailed protocol for mass cytometry staining, if mass cytometry is not available, measurement of cells at the end of this protocol can be done with an alternative single-cell profiling method with sufficient depth to capture the relevant immune populations, such as spectral flow cytometry.
Materials and reagents
Biological materials
1. Human PBMCs from healthy donors (collected under local ethical approval or purchased from Stem Cell Technologies, catalog number: 70025.2)
Reagents
1. RPMI 1640 (Nacalai Tesque, catalog number: 30263-95)
2. Fetal bovine serum (FBS), heat-inactivated (Gibco, catalog number: 10437028)
3. Penicillin-Streptomycin-Glutamine (P/S-L-glu) (Thermo Fisher Scientific, catalog number: 10378016)
4. Ultra-LEAFTM purified anti-human CD3 antibody (BioLegend, clone UCHT1, catalog number: 300438)
5. Recombinant human IL-2 (Shionogi, catalog number: Imunace35)
6. Ipilimumab biosimilar (BioXCell, catalog number: SIM0004)
7. Human IgG1 isotype control (BioXCell, catalog number: BE0297)
8. Tazemetostat (Selleck Chemicals, catalog number: S7128)
9. DMSO (Sigma, catalog number: D6250)
10. Pierce Universal nuclease (Thermo Fisher Scientific, catalog number: 88702)
11. CellTrace CFSE Cell Proliferation kit (Invitrogen, catalog number: C34554)
12. EasySepTM Release Human CD4 Positive Selection kit (Stem Cell Technologies, catalog number: 17752)
13. PBS (Nacalai Tesque, catalog number: 14249-24)
14. EDTA (Nacalai Tesque, catalog number: 06894-14)
15. eBioscienceTM Foxp3/Transcription Factor Staining Buffer set (eBioscience, catalog number: 00-5523-00)
16. PierceTM 16% formaldehyde (w/v), methanol-free (Thermo Scientific, catalog number: 28906)
17. Cell acquisition solution (Standard BioTools, catalog number: 201240)
18. EQ four element calibration beads (Standard BioTools, catalog number: 201078)
19. BSA (Sigma, catalog number: A7906)
20. 10× PBS (Rockland, catalog number: MB-008)
21. FACS sorting antibodies:
a. Anti-CD45RA, BV711 (BioLegend, clone HI100, catalog number: 304138, titration: 1:20)
b. Anti-CD4, APC-Cy7 (BD, clone RPA-T4, catalog number: 557871, titration: 1:20)
c. Anti-CD127, AF647 (BioLegend, clone A019D5, catalog number: 351318, titration: 1:20)
d. Anti-CXCR5, BV421 (BD, clone RF8B2, catalog number: 562747, titration: 1:20)
e. Anti-CD45RO, PerCP-Cy5.5 (BD, clone UCHL1, catalog number: 560607, titration: 1:20)
f. Anti-CD25, PE (BD, clone M-A251, catalog number: 555432, titration: 1:5)
g. Anti-CD19, V500 (BD, clone HIB19, catalog number: 561121, titration: 1:20)
22. Mass cytometry antibodies: all self-conjugated antibodies (all aside from Standard BioTools) were conjugated as previously described [19,20] and stored at a stock concentration of 0.5 mg/mL unless otherwise stated
Barcoding and viability
a. Human Fc block (BioLegend, catalog number: 422302, titration: 1:50)
b. Anti-CD45, 89Y (Standard BioTools, clone HI30, catalog number: 3089003B, titration: 1:1:50)
Note: Labels such as 89Y refer to the specific metal isotopes used for mass-tagging antibodies, where the number (89) indicates the mass number of the isotope and the letter (Y) represents the chemical element (yttrium in this case). These isotopes act as unique tags detected by the mass cytometer, allowing simultaneous quantification of many markers. This naming convention is consistently applied throughout the manuscript.
c. Anti-CD45, 113In/115In/194Pt/195Pt/196Pt/198Pt (BioLegend, clone HI30, catalog number: 304002, titration: 1:100)
d. DCED Palladium (Sigma, catalog number: 574902, stock concentration: 500 μM, titration: 1:500, final concentration: 1 μM)
e. Alternative viability dye: Cell-IDTM Cisplatin-198Pt (Standard BioTools, catalog number: 201198, titration: 1:2,000)
Pre-stain
Pre-stain contains antibodies that stain better at 37 °C as well as de novo biosynthesis tagging reagents
a. Anti-CD45RA, 153Eu (BioLegend, clone HI100, catalog number: 304102, titration: 1:100)
b. Anti-CXCR3, 154Sm (BioLegend, clone G025H7, catalog number: 353733, titration: 1:100)
c. Anti-CCR7, 155Gd (BioLegend, clone G043H7, catalog number: 353202, titration: 1:100)
d. Anti-CCR4, 160Gd (BioLegend, clone L291H4, catalog number: 359402, titration: 1:100)
e. Anti-CCR6, 163Dy (BioLegend, clone G034E3, catalog number: 353427, titration: 1:50)
f. Anti-CXCR5 (BD, clone RF8B2, catalog number: 552118, titration: 1:25)
g. Puromycin (Sigma, catalog number: P8833, concentration: 1 mg/mL, titration: 1:300)
h. BrU (Sigma, catalog number: 850187, concentration: 5 mM, titration: 1:20)
i. IdU (Standard BioTools, catalog number: 201192A, concentration: 125 μM, titration: 1:600)
Surface stain
a. Anti-CD21, 110Cd (BioLegend, clone Bu32, catalog number: 354902, titration: 1:100)
b. Anti-CD4, 111Cd (BioLegend, clone RPA-T4, catalog number: 300502, titration: 1:100)
c. Anti-CD8, 112Cd (BioLegend, clone RPA-T8, catalog number: 301002, titration: 1:50)
d. Anti-CD39, 114Cd (BioLegend, clone A1, catalog number: 328221, titration: 1:100)
e. Anti-CD19, 116Cd (BioLegend, clone HIB19, catalog number: 302202, titration: 1:100)
f. Anti-CD86, 147Sm (BioLegend, clone IT2.2, catalog number: 305402, titration: 1:50)
g. Anti-IgD, 151Eu (BioLegend, clone IA6-2, catalog number: 348202, titration: 1:100)
h. Anti-PD-L1 (CD274), 156Gd (Standard BioTools, clone 29E.2A3, catalog number: 3156026B, titration: 1:100)
i. Anti-CD27, 158Gd (Standard BioTools, clone L128, catalog number: 3158010B, titration: 1:200)
j. Anti-TIM3, 159Tb (Standard BioTools, clone F38-2E2, catalog number: 3159037B, titration: 1:100)
k. Anti-Biotin (for CXCR5), 165Ho (Standard BioTools, clone 1D4C5, catalog number: 3165012B, titration: 1:50)
l. Anti-PD1, 166Er (BioLegend, clone EH12.2H7, catalog number: 329941, titration: 1:100)
m. Anti-CD25, 169Tm (Standard BioTools, clone 2A3, catalog number: 3169003B, titration: 1:200)
n. Anti-CD98, 171Yb (BD, clone UM7F8, catalog number: 556074, titration: 1:200)
o. Anti-CD38, 172Yb (Standard BioTools, clone HIT2, catalog number: 3172007B, titration: 1:100)
p. Anti-CD11c, 173Yb (BioLegend, clone S-HCL-3, catalog number: 371502, titration: 1:100)
q. Anti-HLA-DR, 174Yb (BioLegend, clone L243, catalog number: 307602, titration: 1:400)
r. Anti-TIGIT, 209Bi (Standard BioTools, clone MBSA43, catalog number: 3209013B, titration: 1:100)
Intracellular stain
a. Anti-H3K27me3, 140Ce (Active Motif, clone 323, catalog number: 61017, titration: 1:200)
b. Anti-Puromycin, 141Pr (Merck, clone 12D10, catalog number: MABE343, titration: 1:200)
c. Anti-Cleaved Caspase-3, 142Nd (Standard BioTools, clone D3E9, catalog number: 3142004A, titration: 1:100)
d. Anti-TCF1, 143Nd (BioLegend, clone 7F11A10, catalog number: 655202, titration: 1:50)
e. Anti-FITC (for CFSE), 144Nd (Standard BioTools, clone FIT-22, catalog number: 3144006B, titration: 1:100)
f. Anti-Helios, 145Nd (BioLegend, clone 22F6, catalog number: 137202, titration: 1:100)
g. Anti-OGDH, 146Nd (Thermo Fisher Scientific, polyclonal, catalog number: PA528195, titration: 1:100)
i. Anti-XBP1, 148Nd (Thermo Fisher Scientific, polyclonal, catalog number: PA5-27650, titration: 1:100)
h. Anti-Cytochrome C, 149Sm (BioLegend, clone 6H2.B4, catalog number: 612302, titration: 1:200)
i. Anti-Granzyme B, 150Nd (Novus, clone CLB-GB11, catalog number: NBP1-50071, titration: 1:100)
j. Anti-CPT1A, 152Sm (Abcam, clone 8F6AE9, catalog number: ab128568, titration: 1:100)
k. Anti-T-bet, 157Gd (BioLegend, clone 4B10, catalog number: 644802, titration: 1:100)
l. Anti-VDAC1/Porin, 161Dy (Abcam, clone 20B12AF2, catalog number: ab14734, titration: 1:100)
m. Anti-Foxp3, 162Dy (eBioscience, clone 236A/E7, catalog number: 14-4777-82, titration: 1:200)
n. Anti-Cyclin B1, 164Dy (Standard BioTools, clone GNS-1, catalog number: 3164010A, titration: 1:200)
o. Anti-GLUT1, 167Er (Abcam, clone EPR3915, catalog number: ab196357, titration: 1:200)
p. Anti-Ki67, 168Er (Standard BioTools, clone Ki67, catalog number: 3168001B, titration: 1:200)
q. Anti-CTLA4, 170Er (Standard BioTools, clone 14D3, catalog number: 3170005B, titration: 1:50)
r. Anti-BrdU (for BrU), 175Lu (BD, clone 3D4, catalog number: 555627, titration: 1:50)
s. Anti-OPA1, 176Yb [Abcam, clone EPR11057(B), catalog number: ab240143, titration: 1:100]
DNA stain
a. Cell-IDTM Intercalator-Rh (103Rh) (Standard BioTools, catalog number: 201103A, titration: 1:500)
Solutions
1. PBSE (see Recipes)
2. Complete RPMI (cRPMI) (see Recipes)
3. cRPMI + DNAse (see Recipes)
4. PBS with 0.1% FBS (see Recipes)
5. CyFACS (see Recipes)
Recipes
Note: PBSE, cRPMI, and PBS with 0.1% FBS solutions are made with sterile ingredients and handled sterile. Especially, myeloid cells depend on the quality of the FBS, and thus, we recommend making new solutions every week.
1. PBSE, 50 mL
49 mL of PBS (should be free of Ca++ and Mg++)
1 mL of FBS (2% final)
100 μL of EDTA (0.5 M stock, 1 mM EDTA final)
2. Complete RPMI (cRPMI), 50 mL
45 mL of RPMI
5 mL of FBS (10% final)
0.5 mL of P/S-L-glu (1% final)
3. cRPMI + DNAse, 50 mL
45 mL of RPMI
5 mL of FBS (10% final)
0.5 mL of P/S-L-glu (1% final)
4 μL of Pierce Universal nuclease stock (stock is 25 kU/100 μL → 250 U/μL; final: 500 U/25 mL)
4. PBS with 0.1% FBS, 10 mL
10 mL of PBS (should be free of Ca++ and Mg++)
10 μL of FBS (1,000× dilution)
5. CyFACS, 500 mL
50 mL of 10× PBS
2 mL of EDTA (0.5 M stock, 2 mM EDTA final)
0.5 g of BSA (0.1% final)
Top up to 500 mL with Milli-Q water. Filter-sterilize with a 0.2 μm filter. Store at 4 °C for up to 1 year.
Laboratory supplies
1. 15 mL and 5 mL round-bottom polystyrene tubes (for magnetic separation and FACS sorting)
2. 96-well round-bottom plates (Nunc, catalog number: 163320)
3. Neubauer chamber (for manual cell counting)
4. Inverted light microscope (for visual well checks)
5. 35 μm cell strainer (BD, catalog number: 352235)
6. Nalgene filters with PES membrane, 0.2 μm pore size, 1,000 mL capacity (Thermo Scientific, catalog number: 567-0020)
Equipment
1. Aria III cell sorter (BD Biosciences)
2. Centrifuge (suitable for 5 and 15 mL tubes with 4 °C option)
3. CO2 incubator (humidified, 37 °C, 5% CO2)
4. Water bath (37 °C)
5. CyTOF Helios (Standard BioTools) or equivalent mass cytometer
6. Standard biological safety cabinet (class II)
Software and datasets
1. CyTOF acquisition software (Standard BioTools, v. 7.0.8493)
2. Cytobank (Beckman Coulter, v. 10.5) - license required
3. R software, v. 4.3.0 - open source
a. Premessa v. 0.3.4
b. CATALYST v. 1.26.0
c. ggplot2 v. 3.4.4
d. diffcyt v. 1.22.0
e. scran v. 1.30.0
f. rstatix v. 0.7.2
4. GitHub repositories:
a. https://github.com/jonasns/scSPOT
b. https://github.com/jonasns/scDivisionPro
5. Zenodo archives:
a. scSPOT dataset, https://doi.org/10.5281/zenodo.10828517
b. scSPOT code, https://doi.org/10.5281/zenodo.14056251
c. scDivisionPro code, https://doi.org/10.5281/zenodo.14064605
Procedure
文章信息
稿件历史记录
提交日期: May 21, 2025
接收日期: Jul 22, 2025
在线发布日期: Aug 1, 2025
出版日期: Aug 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Søndergaard, J. N., Tulyeu, J., Priest, D., Sakaguchi, S. and Wing, J. B. (2025). Assessing Human Treg Suppression at Single-Cell Resolution Using Mass Cytometry. Bio-protocol 15(16): e5424. DOI: 10.21769/BioProtoc.5424.
分类
免疫学 > 免疫机理 > 体外模型
细胞生物学 > 细胞分离和培养 > 共培养
免疫学 > 免疫细胞染色 > 大量细胞计数法
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link