- EN - English
- CN - 中文
Rapid Isolation and Flow Cytometry Analysis of Murine Intestinal Immune Cells After Chemically Induced Colitis
化学诱导结肠炎后小鼠肠道免疫细胞的快速分离与流式细胞分析
发布: 2025年08月20日第15卷第16期 DOI: 10.21769/BioProtoc.5417 浏览次数: 3140
评审: Anonymous reviewer(s)
Abstract
Chemically induced murine colitis models are widely used to understand intestinal homeostasis and inflammatory responses during acute and chronic gut inflammation, such as inflammatory bowel disease (IBD). Resident populations of immune cells, together with those recruited during an inflammatory response, maintain intestinal immunity by mounting an effective immune response to enteropathogenic microbes while at the same time maintaining tolerance against commensals. To better understand the disease mechanism, studying different immune cell populations and their dynamic changes during infection and inflammation is essential. However, isolating healthy and viable immune populations, particularly hyperactivated neutrophils and macrophages from the inflamed gut (i.e., active disease site), is challenging as tissues are usually subjected to rigorous enzymatic digestion for an extended period. Here, we describe a method that uses a cell dissociator (Medimachine II from Syntec International) to separate intestinal tissue after short enzymatic digestion to obtain a single-cell suspension. This technique facilitates the isolation of immune cells from mouse intestinal tissues in high quantity and with superior viability in a very short time frame. This protocol delivers 80%–90% cell viability, which is 1.5 to 2-fold higher than conventional methods of isolating cells from inflamed mouse colons. The composition, phenotype, activation state, and gene expression profile of cells isolated using this protocol can be assessed by using multiple methods, including, but not limited to, flow cytometry, quantitative PCR, immunoblotting, mass spectrometry, single-cell RNA sequencing, and functional readouts such as reactive oxygen species (ROS) production.
Key features
• Infiltrating immune cells are key drivers of intestinal inflammation.
• Isolating viable cells from the inflamed colon is slow and may alter cell activation and expression profiles.
• This protocol enables faster isolation with improved cell viability.
• Isolated cells can be further purified using magnetic beads or flow cytometry for downstream analysis.
Keywords: Neutrophils (中性粒细胞)Graphical overview
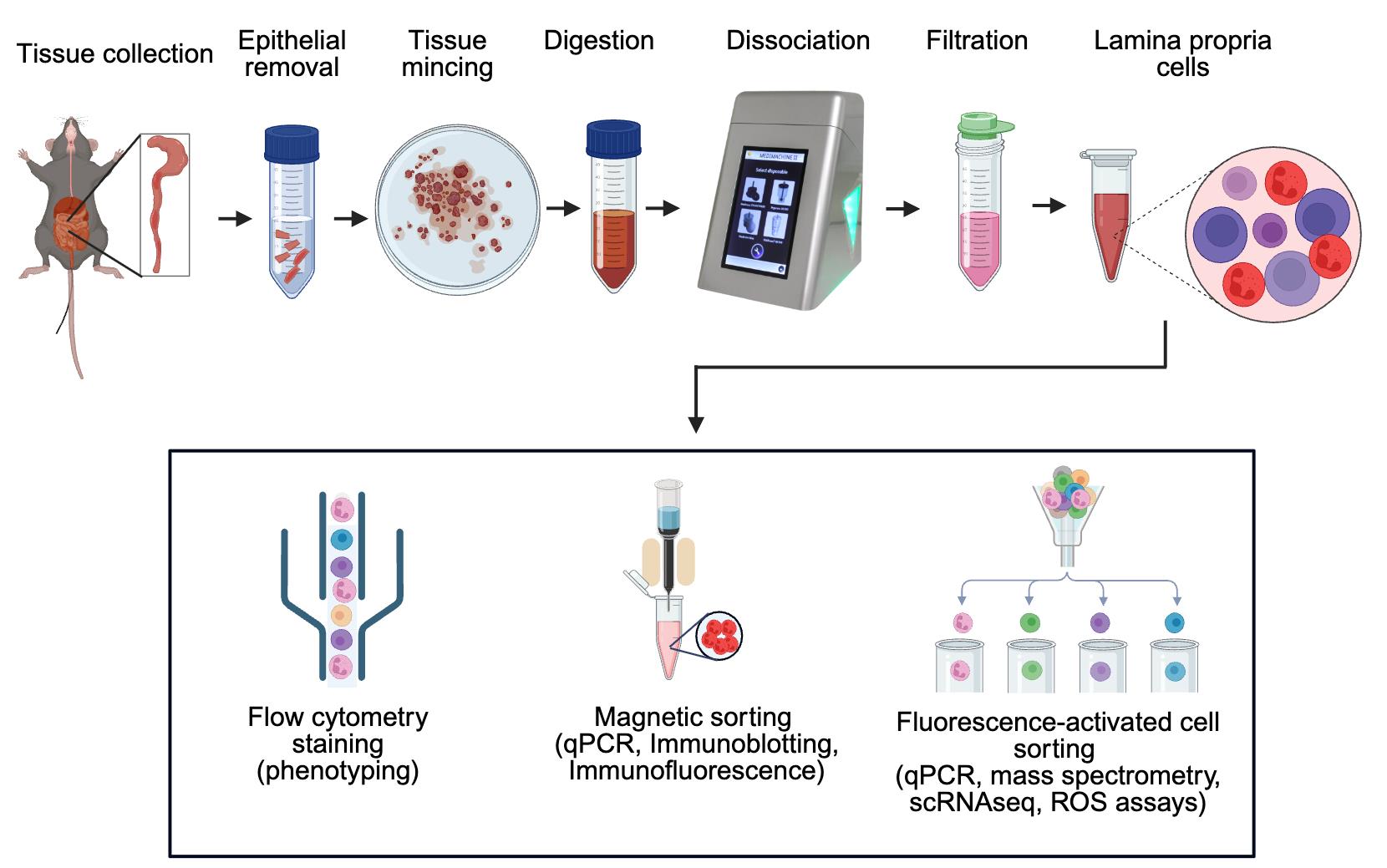
Workflow for efficient isolation of murine intestinal immune cells via combined enzymatic and mechanical dissociation
Background
An intricate network of various immune cells and secreted cytokines and chemokines functions in cooperative ways to protect the host from adverse environmental conditions and microbial infections, to maintain or restore homeostasis [1]. The gastrointestinal (GI) tract is constantly exposed to commensal, opportunistic, and pathogenic microbes, creating perturbations that can lead to life-threatening infections and chronic inflammation. The host defense system in the GI tract is comprised of a single layer of epithelium fortified with adjoining cells and lymphoid tissues that operate efficiently to maintain barrier homeostasis. This barrier strikes a balance of remaining tolerant to commensal microbes and dietary antigens while preserving the capacity to mount an inflammatory response against pathogens.
Inflammatory bowel disease (IBD), a general term for chronic inflammatory conditions of the GI tract, is comprised of two main clinical entities: Crohn’s disease and ulcerative colitis. A broadly compromised or dysregulated immune system due to environmental changes, genetic background, or a qualitative and quantitative abnormal gut microbiota is a hallmark of IBD pathogenesis [2]. Despite several decades of research, our understanding of onset, recovery, and recurrent flares in IBD remains tenuous. Recruitment and activation of immune cells at inductive sites [Peyer’s patches (PP), isolated lymphoid follicles (IELs), and mesenteric lymph nodes (MLNs)] and initiation of effector functions in the lamina propria and epithelium is a sequential and complex process. In chronic disease conditions, constant activity of immune cells (i.e., ROS generation, chemokine, and cytokine secretion) in conjunction with architectural damage of tissues further induces immune cell recruitment, hence forming a perpetual destructive loop [3]. To fully comprehend the spatiotemporal dynamics of transmigrated cells and their crosstalk with other cell types, efficient isolation and characterization of distinct immune cell populations is essential. A relatively simple and rapid protocol for isolating immune cells from intestinal tissue will be highly beneficial, as intestinal immunity is at the center stage in IBD, colorectal cancer (CRC), or host–microbe interactions. Isolating healthy and viable cells from the inflamed gut, including hyperactivated neutrophils and macrophages in early acute inflammation or various T lymphocytes at later stages of the disease, is challenging but crucial for immunophenotyping and functional assays of selected cell populations. The majority of the existing protocols describe isolation of cells from normal (non-inflamed) tissues during homeostasis [4–6]; however, those that isolate from inflamed tissues have an extended digestion period and often lack viability data [7–10].
This protocol describes a straightforward method for rapid isolation of highly viable immune cells from murine intestinal tissues, such as colonic and ileal lamina propria, PP, and MLN, using Medimachine II (Syntec International, Ireland). This protocol has also been used to isolate viable cells from the murine spleen and can easily be adapted to phenotype diverse cell populations from human tissues. Here, we used chemically induced models of acute colitis in mice, such as 2,4,6-trinitrobenzene sulfonic acid (TNBS) and dextran sodium sulfate (DSS) treatment, for isolating cells from colonic lamina propria at the peak of disease.
Materials and reagents
Biological materials
1. C57BL/6J mice (The Jackson Laboratory, USA)
Reagents
1. 70% industrial methylated spirit (70% IMS)
2. Anti-mouse CD45, Brilliant Violet 510 antibody (BioLegend, clone 30F11, catalog number: 103137)
3. Anti-mouse CD11b, APC (BioLegend, clone M1/70, catalog number: 101211)
4. Anti-mouse Ly6G, PE antibody (BioLegend, clone 1A8, catalog number: 127607)
5. Collagenase D (Sigma-Aldrich, catalog number: 11088866001), stock 500 mg/10 mL HBSS
6. Dispase (Corning, catalog number: 354235), stock 5,000 U/100 mL HBSS
7. DNase I (Sigma-Aldrich, catalog number: 10104159001), stock 100 mg/10 mL HBSS
8. DPBS, calcium and magnesium-free (Thermo Fisher Scientific, catalog number: 14190144)
9. EDTA, 500 mM in H2O, sterile filtered (Sigma-Aldrich, catalog number: E4884-500G)
10. FBS, heat-inactivated (Sigma-Aldrich, catalog number: F7524-500ML)
11. HBSS, calcium and magnesium-free (Thermo Fisher Scientific, catalog number: 14175095)
12. HEPES solution (Sigma-Aldrich, catalog number: H0887-100ML), 1 M
13. LiberaseTM (Sigma-Aldrich, catalog number: 5401119001), stock 5 mg/2 mL HBSS
14. Non-essential amino acids (Sigma-Aldrich, catalog number: M7145-100ML), 100×
15. Sodium azide (NaN3), 100 mM solution in sterile water (Sigma-Aldrich, catalog number: S2002-25G)
16. Penicillin-streptomycin (Thermo Fisher Scientific, catalog number: 15140122), 10,000 U/mL
17. Red blood cell (RBC) lysis buffer (Thermo Fisher Scientific, catalog number: 00-4333-57)
18. RPMI-1640 with L-glutamate, without phenol red (Gibco, catalog number: 11835-063)
19. Sodium pyruvate (Sigma-Aldrich, catalog number: S8636-100ML), 100 mM
20. Trypan Blue solution (Sigma-Aldrich, catalog number: T8154-20 ML)
21. ViaKrome 808 Fixable Viability Dye (Beckman Coulter, catalog number: C36628)
22. Fixable Viability Dye eFluorTM 780 (Thermo Fisher Scientific, catalog number: 65-0865-14)
23. Anti-Mouse CD16/CD32 (Mouse BD Fc BlockTM) (BD Biosciences, catalog number: 553142)
24. eBioscienceTM intracellular (IC) fixation buffer (Thermo Fisher, catalog number: 00-8222-49)
Solutions
1. Epithelial removal solution (see Recipes)
2. Washing solution (see Recipes)
3. 2× Digestion solution (see Recipes)
4. cRPMI (see Recipes)
5. Digestion solution (see Recipes)
6. Flow cytometry staining buffer/FACS buffer (see Recipes)
Recipes
1. Epithelial removal solution (500 mL)
Store at 4 °C for 2–3 weeks. Prewarm in the water bath at 37 °C before use.
| Components | Volume required | Final concentration |
|---|---|---|
| 1 M HEPES solution | 5 mL | 10 mM |
| 500 mM EDTA | 5 mL | 5 mM |
| Penicillin-Streptomycin | 1 mL | 50 U/mL |
| HBSS | 489 mL | -- |
2. Washing solution (500 mL)
Store at 4 °C for 2–3 weeks.
| Components | Volume required | Final concentration |
|---|---|---|
| 1 M HEPES | 5 mL | 10 mM |
| Penicillin-Streptomycin | 1 mL | 50 U/mL |
| HBSS | 494 mL | -- |
3. 2× Digestion solution (250 mL)
Store at 4 °C for 2–3 weeks.
| Components | Volume required | Final concentration |
|---|---|---|
| 100× non-essential amino acids | 5 mL | 1× |
| 100 mM sodium pyruvate | 5 mL | 2 mM |
| 1 M HEPES | 5 mL | 20 mM |
| FBS (heat-inactivated) | 12.5 mL | 10% |
| Penicillin-Streptomycin | 1 mL | 100 U/mL |
| RPMI-1640 | 221.5 mL | -- |
4. cRPMI (600 mL)
Store at 4 °C for 2–3 weeks.
| Components | Volume required | Final concentration |
|---|---|---|
| 100× non-essential amino acids | 6 mL | 1× |
| 100 mM sodium pyruvate | 6 mL | 1 mM |
| 1 M HEPES | 6 mL | 10 mM |
| FBS (heat-inactivated) | 60 mL | 10% |
| Penicillin-Streptomycin | 1.2 mL | 50 U/mL |
| RPMI-1640 | 520.8 mL | -- |
5. Digestion solution (10 mL)
To be made fresh. Keep on ice until use. Prewarm in the water bath at 37 °C before use
| Components | Volume required | Final concentration |
|---|---|---|
| Dispase | 2.5 mL | 12.5 U/mL |
| Collagenase D | 50 μL | 250 μg/mL |
| LiberaseTM | 150 μL | 37.5 μg/mL |
| DNase I | 200 μL | 200 μg/ mL |
| 2× Digestion solution (Recipe 3) | 5 mL | 1× |
| RPMI-1640 | 2.1 mL | -- |
6. Flow cytometry staining buffer/FACS buffer (100 mL)
| Components | Volume required | Final concentration |
|---|---|---|
| FBS (heat-inactivated) | 2 mL | 2% |
| EDTA (500 mM) | 0.4 mL | 2 mM |
| NaN3 (100 mM) | 2 mL | 2 mM |
| DPBS | 95.6 mL | -- |
Laboratory supplies
1. 1 and 10 mL syringes (sterile)
2. 100, 70, and 40 μm cell strainers (sterile)
3. 15 and 50 mL tubes (sterile, without skirts)
4. 6-well sterile tissue culture plates (Fisher Scientific, catalog number: 10578911)
Equipment
1. Flow cytometer (Beckman Coulter, model: CytoFLEX LX or any other compatible cytometer)
2. Light microscope
3. Medimachine II® (Syntec International)
4. Swing-bucket Eppendorf® cooling centrifuge
5. Tissue culture incubator set to 37 °C
6. Water bath set to 37 °C
7. Vortex mixer
8. Hemacytometer (Sigma-Aldrich, catalog number: Z359629-1EA)
9. Mouse dissecting tools
10. Medicons and Medicons Max (Syntec International, catalog number: 79500 S)
Procedure
文章信息
稿件历史记录
提交日期: May 28, 2025
接收日期: Jul 10, 2025
在线发布日期: Jul 24, 2025
出版日期: Aug 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Singh, A. K., Blanco, A., Sinnott, R. and Knaus, U. G. (2025). Rapid Isolation and Flow Cytometry Analysis of Murine Intestinal Immune Cells After Chemically Induced Colitis. Bio-protocol 15(16): e5417. DOI: 10.21769/BioProtoc.5417.
分类
免疫学 > 免疫细胞分离 > 嗜中性粒细胞
免疫学 > 免疫细胞染色 > 流式细胞术
细胞生物学 > 基于细胞的分析方法 > 炎症反应
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link