- EN - English
- CN - 中文
Transplantation of Cultured Myoblasts Into Intact Skeletal Muscle and Analysis of Muscle Contraction Force in Mice Model
培养的肌母细胞移植至完整骨骼肌及小鼠模型肌肉收缩力分析
发布: 2025年08月20日第15卷第16期 DOI: 10.21769/BioProtoc.5413 浏览次数: 1797
评审: Xiaokang WuIshita ChandelAnonymous reviewer(s)
Abstract
Cell transplantation is a promising strategy for treating age-related muscle atrophy, but its critical application remains limited. Cultured myoblasts, unlike freshly isolated muscle stem cells, show poor engraftment efficiency and fail to contribute effectively to muscle regeneration. Moreover, successful engraftment generally requires prior muscle injury, as skeletal muscle regeneration is typically triggered by a damaged microenvironment. These limitations present major obstacles for applying cell therapy to sarcopenia, where muscle degeneration occurs without injury. In this protocol, we describe a novel approach that enables the transplantation of cultured myoblasts into intact skeletal muscle without the need for preexisting injuries or genetic modification. By combining myoblasts with extracellular matrices (ECM), such as Matrigel, which mimic the native muscle niche and support cell survival, adhesion, proliferation, and differentiation, we achieve efficient engraftment and increased muscle mass without the need for preexisting injury. The ECM also provides a scaffold and retains bioactive factors that enhance the regenerative capacity of transplanted cells. This is the first protocol that enables robust myoblast engraftment in non-injury muscle conditions, offering a practical tool for studying and potentially treating sarcopenia.
Key features
• Cultured myoblasts mixed with extracellular matrix components are transplanted into intact skeletal muscle.
• Contraction force measurement of the tibialis anterior muscle in vivo.
• Cell transplantation without muscle injury would be applied for the treatment of sarcopenia.
Keywords: Skeletal muscle (骨骼肌)Graphical overview
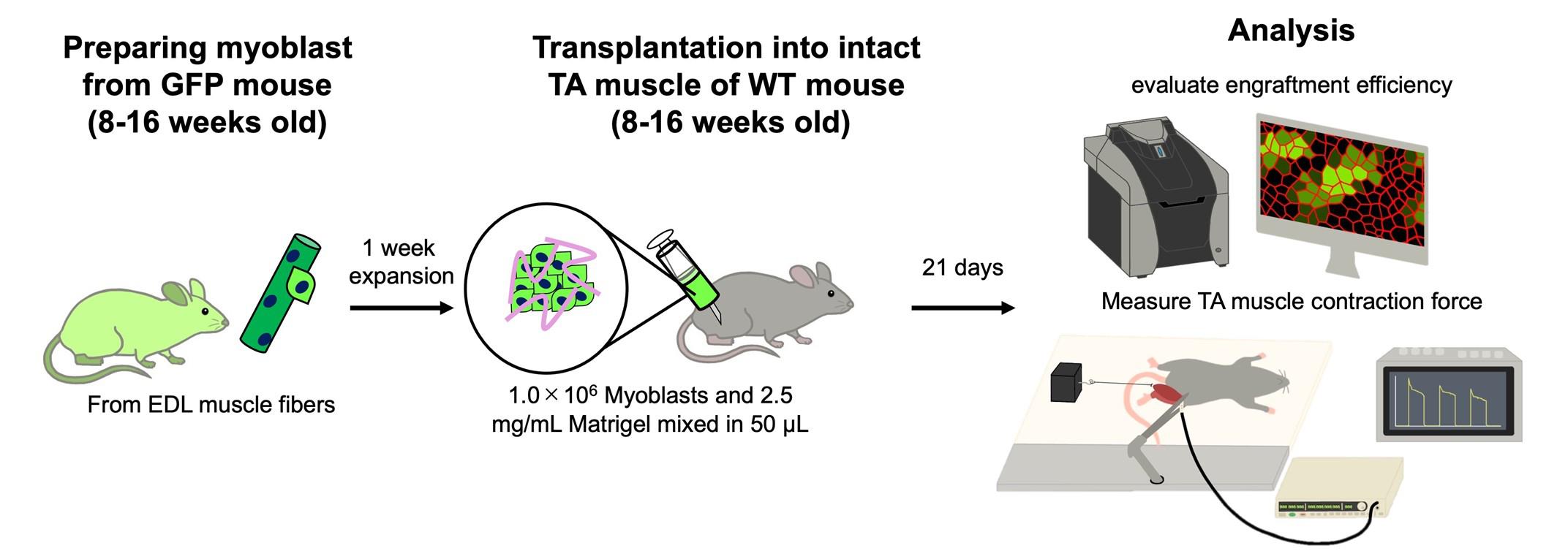
Background
Age-related muscle atrophy is a major global concern, contributing to reduced quality of life and life expectancy. One promising approach to treat skeletal muscle atrophy is cell transplantation using muscle progenitor cells. Skeletal muscle has a high potential to regenerate itself, and muscle stem cells called satellite cells contribute to myogenesis by responding to damaged muscle cells (myofibers). Upon myofibers being damaged, satellite cells activate into myoblasts, and they begin proliferation and differentiation into myofibers. This regenerative potential of myoblasts forms the basis for cell-based therapies targeting skeletal muscle disorders. However, since the first attempt to repair muscle tissue through myoblast transplantation [1], the low efficiency of cell engraftment has been a challenge. Satellite cells are limited in number and need to be expanded in vitro as myoblasts to achieve enough numbers for cell transplantation [2,3], while myoblasts being cultured ex vivo show low transplantation efficiency, and most of the transplanted cells cannot contribute to myogenesis and are eliminated [4–6].
Another issue in achieving cell therapy for age-related skeletal muscle atrophy is that the transplanted cells rarely engraft into intact tissue [4,6,7]. Inducing injury to the muscle tissue is considered an essential step for successful cell engraftment [8,9] because the microenvironment (niche) in damaged muscle promotes myogenesis. Many types of cells and secreted factors accelerate muscle regeneration, including the activation of satellite cells or the proliferation and differentiation of myoblasts [3,10,11]. In the intact tissue, satellite cells stay in a quiescent state, and myogenesis is not conducted. Transplanted cells cannot create myofibers in this niche. From these findings, many researchers consider that cell therapy is hardly available for the treatment of sarcopenia, which does not show a significant collapse of myofibers.
We have recently established a method to successfully engraft cultured myoblasts into intact skeletal muscle tissue [12]. Our approach focused on creating a supportive niche that allows myoblasts to proliferate and differentiate. In general, extracellular matrix (ECM) components must be coated for the adhesion of myoblasts to the culture dishes, and ECM also plays a critical role in in vivo muscle regeneration, which promotes the activation of satellite cells and myoblast proliferation and differentiation [3,10,11]. We mix myoblasts and Matrigel, which are widely used as ECM components, and transplant them into intact muscle. We found that the myoblasts injected with Matrigel exhibited enhanced engraftment efficiency, and muscle weight was increased by transplantation [12]. This article provides a detailed protocol for myoblast transplantation and functional analysis of the transplanted tibialis anterior muscle. A limitation of our method is that transplanted myoblasts generate both mature and immature myofibers without enhancing muscle contraction force. Methodological improvements are needed to promote myofiber maturation and increase muscle strength.
Materials and reagents
Biological materials
1. C57BL/6 wild-type mice (8–16 weeks old, from Japan SLC, Inc.)
2. CAG-EGFP mice (8–16 weeks old, from Japan SLC, Inc.)
Reagents
1. Type 1 collagenase (Worthington Biochemical Corporation, catalog number: LS004196)
2. DMEM GlutaMAX (Gibco, catalog number: 10569044)
3. Penicillin-Streptomycin-Amphotericin B suspension (Fujifilm Wako, catalog number: 161-23181)
4. Bovine serum albumin (BSA) (Sigma, catalog number: A9418)
5. Phosphate-buffer saline (PBS) (Gibco, catalog number: 18912014)
6. Accutase (Innovative Cell Technologies, catalog number: AT104)
7. Matrigel (Corning, catalog number: 354230)
8. DMEM, no glucose, no glutamine, no phenol red (Gibco, catalog number: A1443001)
9. Fetal bovine serum (FBS) (Gibco, catalog number: 10437-028)
10. GlutaMAX (Thermo Fisher, catalog number: 35050061)
11. Chicken embryo extract (USBiological Life Sciences, catalog number: C3999)
12. FGF-basic, murine, recombinant (Pepro Tech Inc., catalog number: 450-33)
13. Trypsin-EDTA (Gibco, catalog number: 25200072)
14. NaCl (Wako, catalog number: 196-01671)
15. Domitor (ZENOAQ, catalog number: AHE1)
16. Mitazolam (Maruishi Pharmaceutical Corporation, catalog number: 1124401A1052)
17. Butorphanol (Meiji Animal Health Corporation, catalog number: VETLI5-B)
18. Anti-anesthetic (ZENOAQ, catalog number: AHC1)
19. Tissue-Tek O.C.T (Sakura Finetek, catalog number: 45833)
20. 4% paraformaldehyde (Nacalai Tesque, catalog number: 09154-85)
21. Goat serum (Vector Laboratories, catalog number: S-1000-20)
22. Laminin-alpha2 antibody (Enzo Life Sciences, catalog number: ALX-804-190, from rat)
23. Alexa Fluor 594 anti-rat (Invitrogen, catalog number: A-11007)
24. VectaShield mounting medium with DAPI (Vector Laboratories, catalog number: H-1200)
25. KCl (Wako, catalog number: 163-03545)
26. CaCl2·2H2O (Wako, catalog number: 035-00455)
27. KH2PO4 (Wako, catalog number: 169-04245)
28. MgSO4·7H2O (Wako, catalog number: 138-00415)
29. NaHCO3 (Wako, catalog number: 191-01305)
30. Disinfectants (Orimatsu, catalog number: WK-Ⅱ-75)
Solutions
1. Myofiber isolation medium (see Recipes)
2. BSA solution (see Recipes)
3. Collagenase solution (see Recipes)
4. Saline (see Recipes)
5. Myoblast growth medium (see Recipes)
6. Myoblast transplantation solution (see Recipes)
7. Anesthetic (see Recipes)
8. Anti-anesthetic (see Recipes)
9. Immunohistochemistry blocking solution (see Recipes)
10. KRB buffer (see Recipes)
Recipes
1. Myofiber isolation medium
Prepare under a clean bench. Add the Penicillin-Streptomycin-Amphotericin B suspension directly to the DMEM GlutaMAX bottle and store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Penicillin-Streptomycin-Amphotericin B suspension | 1.0% | 5.0 mL |
| DMEM GlutaMAX (500 mL bottle) | 500 mL |
2. BSA solution
Add 5 g of BSA powder to 80 mL of PBS. The final volume will be approximately 100 mL after dissolution. After dissolving BSA, inactivate at 60 °C for 30 min. After the solution cools down at room temperature (RT), separate the solution into approximately 10 mL in a 25 mL tube using a 0.22-μm filter and store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 5.0% | 5.0 g |
| PBS | 80 mL |
3. Collagenase solution
Dissolve collagenase in a 5 mL tube and sterilize with a 0.22-μm filter. Transfer the solution to a 15 mL tube for the myofiber incubation. Use 2.5 mL per two extensor digitorum longus (EDL) muscles from one mouse. Warm to 37 °C before use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Type 1 collagenase | 0.25% | 6.25 mg |
| Myofiber isolation medium | 2.5 mL |
4. Saline
Make in a glass bottle and autoclave at 121 °C for 20 min. Store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 0.9% | 0.9 g |
| H2O | 99.1% | 80 mL |
| Total | Adjust the final volume with H2O | 100 mL |
5. Myoblast growth medium
Store the medium at 4 °C. We recommend using it within 1 week [13]. Warm the medium at 37 °C before use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| FBS | 30.0% | 30.0 mL |
| Chicken embryo extract | 1.0% | 1.0 mL |
| Penicillin-Streptomycin-Amphotericin B suspension | 1.0% | 1.0 mL |
| Basic-FGF (stock: 50 µg/mL) | 10 ng/mL | 20.0 μL |
| GlutaMAX | 1.0% | 1.0 mL |
| No glucose DMEM | 67.0% | 67.0 mL |
| Total | 100 mL |
6. Myoblast transplantation solution
Prepare on ice as the original concentration of Matrigel easily gellifies at RT.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Matrigel (original: 8.2 mg/mL) | 2.5 mg/mL | 30.49 μL |
| DMEM GlutaMAX | 19.51 μL | |
| Saline | 50.00 μL | |
| Total | 100 μL |
7. Anesthetic
Use sterilized saline, store at 4 °C, and cover with aluminum foil to block the light.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Domitor | 1.0 mg/mL | 0.75 mL |
| Mitazolam | 5.0 mg/mL | 2.00 mL |
| Butorphanol | 5.0 mg/mL | 2.50 mL |
| Saline | 7.25 mL | |
| Total | 12.50 mL |
8. Anti-anesthetic
Use sterilized saline, store at 4 °C, and cover with aluminum foil to block the light.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Anti-anesthetic | 5.0 mg/mL | 1.20 mL |
| Saline | 8.80 mL | |
| Total | 10.0 mL |
9. Immunohistochemistry blocking solution
Store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Goat serum | 20.0% | 400 μL |
| PBS | 1,600 μL | |
| Total | 2.0 mL |
10. KRB buffer
Prepare each stock solution in advance and store at 4 °C. Mix the reagents in co-washed containers. Make fresh and warm at 37 °C before use.
Caution: If it contains impurities, the liquid turns white.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 5.0 M NaCl | 117 mM | 0.171 g |
| 1.0 M KCl | 4.7 mM | 118 μL |
| 1.0 M CaCl2·2H2O | 2.5 mM | 63 μL |
| 0.5 M KH2PO4 | 1.2 mM | 60 μL |
| 0.5 M MgSO4·7H2O | 1.2 mM | 60 μL |
| 1.0 M NaHCO3 | 24.6 mM | 0.052 g |
| Total | Adjust the final volume with H2O | 25 mL |
Laboratory supplies
1. 0.22 μm filter (MERCK, catalog number: SLGVR33RB)
2. Glass Pasteur pipette (Iwaki, catalog number: IK-PAS-5P)
3. 100 mm cell culture dishes (Iwaki, catalog number: 3020-100)
4. 50 mm deep dishes (Thermo Fisher, catalog number: 124-17)
5. Serological pipettes, 5 mL (FastGene, catalog number: FG3007)
6. Serological pipettes, 10 mL (FastGene, catalog number: FG3008)
7. Serological pipettes, 25 mL (FastGene, catalog number: FG3009)
8. Serological pipettes, 50 mL (FastGene, catalog number: FG3010)
9. Pipettes (SARTORIUS, catalog number: LH-729010/20/30/50/60/70)
10. 10 μL filter pipette tips (BMBio, catalog number: WEF-10RS)
11. 100 μL filter pipette tips (BMBio, catalog number: WEF-100RS)
12. 200 μL filter pipette tips (BMBio, catalog number: WEF-200RS)
13. 1,250 μL filter pipette tips (NICHIRYO, catalog number: 00-LRT-F1250RB)
14. 1.5 mL tubes (Watson, catalog number: 131-715C)
15. 5.0 mL tubes (SANSYO Co., Ltd, catalog number: ST-500)
16. 15 mL centrifuge tubes (Iwaki, catalog number: 2325-015N)
17. 25 mL centrifuge tubes (Iwaki, catalog number: 2362-025N)
18. 50 mL centrifuge tubes (Iwaki, catalog number: 2345-050N)
19. 100 mL centrifuge tubes (Iwaki, catalog number: 2355-100N)
20. Cell counter (OneCell, catalog number: 2-7124-02)
21. Surgical tape (NICHIBAN, catalog number: No. 25)
22. Twenty-six-gauge, 1 mL capacity plastic syringe (JMS Co., Ltd., catalog number: JS-VM2613R)
23. CO2 gas (Nissan TANAKA, catalog number: 87799)
24. APS coated slide glass (Matsunami, catalog number: 83-1631)
25. Cork stand (Uchiyama Manufacturing Corp., catalog number: 56735254)
26. Cover glass (Matsunami, catalog number: 83-0223/83-0225)
Equipment
1. Ultrapure water production equipment (SARTORIUS, catalog number: H2O mm-T)
2. High-pressure steam sterilizer (TOMY, catalog number: LPS-300)
3. Stereomicroscope (Leica, catalog number: M165 C)
4. Bio clean bench (PHCbi, catalog number: MCV-B131F)
5. Bio-shaker (TAITEC, catalog number: BR-40LF)
6. CO2 incubator (PHCbi, catalog number: MCO-170AICUVD)
7. Inverted microscope (EVIDENT, catalog number: CKX53)
8. Centrifuge for 15 mL tubes (HERMLE, catalog number: Z 207 A)
9. Centrifuge for 1.5 mL tubes (KUBOTA, catalog number: 3520)
10. Cryostat (Leica, catalog number: CM 1950)
11. Fluorescence microscope (KEYENCE, catalog number: BZ-810)
12. Mouse muscle tension measurement system (Uchida Denshi)
13. Electronic stimulator (Nihon Kohden, catalog number: SEN-3401)
Software and datasets
1. KEYENCE BZ-X series Image Analyzer (KEYENCE, version: BZ-X800_Analyzer.exe, 1.1.2.4)
2. ImageJ-Fiji (Version: 2.16.0/1.54p)
Procedure
文章信息
稿件历史记录
提交日期: Apr 27, 2025
接收日期: Jul 10, 2025
在线发布日期: Jul 29, 2025
出版日期: Aug 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Dohi, K. and Furuichi, Y. (2025). Transplantation of Cultured Myoblasts Into Intact Skeletal Muscle and Analysis of Muscle Contraction Force in Mice Model. Bio-protocol 15(16): e5413. DOI: 10.21769/BioProtoc.5413.
分类
细胞生物学 > 细胞移植 > 同系移植
细胞生物学 > 组织分析 > 电生理学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












