- EN - English
- CN - 中文
Isolation and Imaging of Microvessels From Brain Tissue
脑组织微血管的分离与成像
发布: 2025年08月05日第15卷第15期 DOI: 10.21769/BioProtoc.5410 浏览次数: 2636
评审: Pilar Villacampa AlcubierreAnonymous reviewer(s)
Abstract
Proper brain function depends on the integrity of the blood–brain barrier (BBB), which is formed by a specialized network of microvessels in the brain. Reliable isolation of these microvessels is crucial for studying BBB composition and function in both health and disease. Here, we describe a protocol for the mechanical dissociation and density-based separation of microvessels from fresh or frozen human and murine brain tissue. The isolated microvessels retain their molecular integrity and are compatible with downstream applications, including fluorescence imaging and biochemical analyses. This method enables direct comparisons across species and disease states using the same workflow, facilitating translational research on BBB biology.
Key features
• The protocol employs mechanical dissociation and density-based separation to isolate microvessels from brain tissues.
• The protocol was used to study molecular changes in brain microvessels in neurodegeneration and aging.
• Validated downstream applications of this method include fluorescence imaging, RNA sequencing, proteomics, western blotting, and ELISA.
• The protocol can be applied to fresh and frozen human and murine brain samples.
Keywords: Vasculature (血管系统)Graphical overview
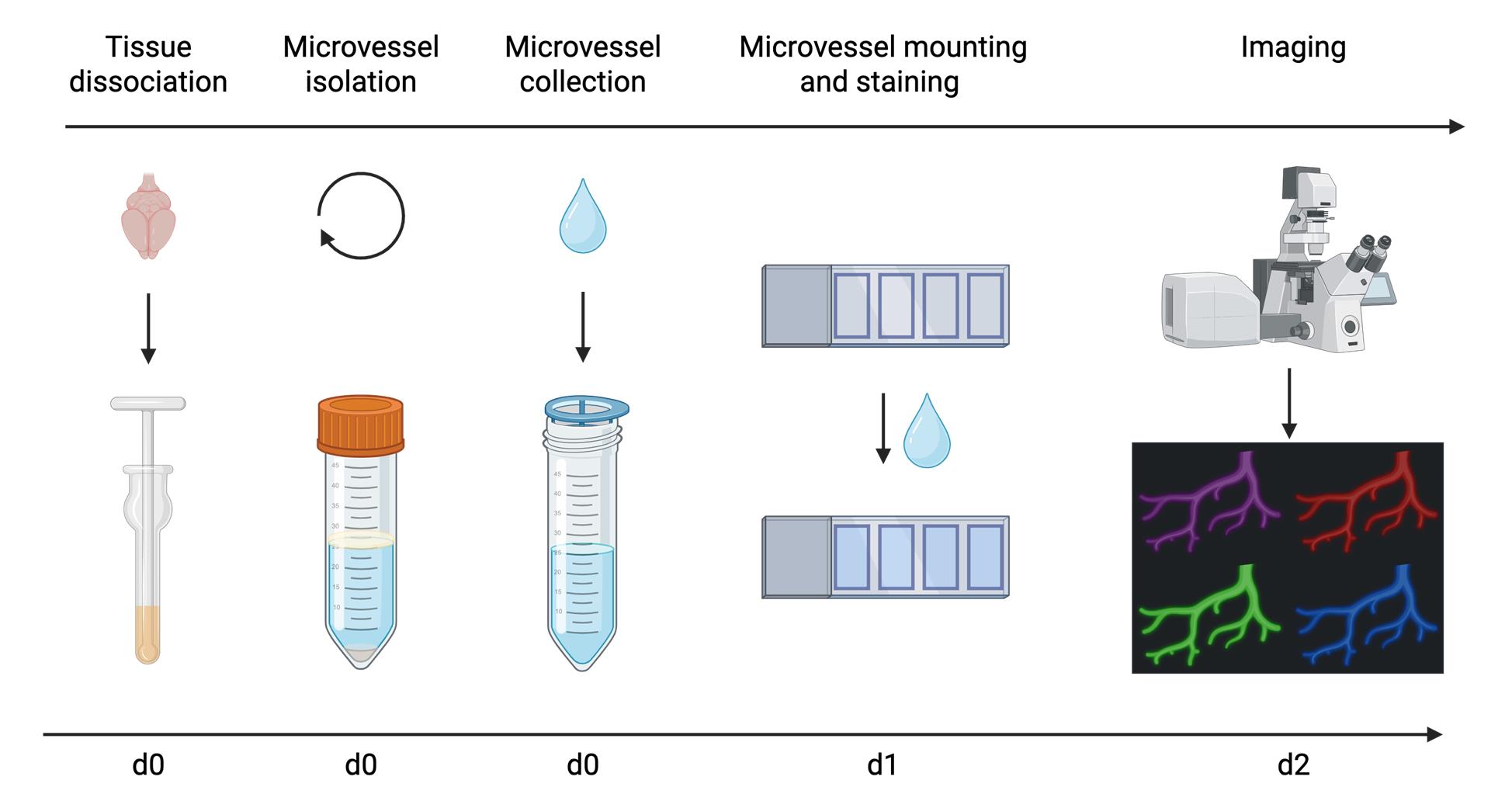
Overview of microvessel isolation and imaging procedure
Background
The blood–brain barrier (BBB) is a highly selective neurovascular interface that tightly regulates the exchange of substances between the blood and brain parenchyma [1]. Its primary physiological function involves maintaining central nervous system (CNS) homeostasis by supplying the brain with necessary nutrients while protecting neural cells from bloodborne pathogens and toxic soluble factors [1–3]. The BBB is formed by different cell types and structural components, including endothelial cells, astrocytic end-feet, pericytes, the basement membrane, and a luminal glycocalyx layer [3,4]. Brain endothelial cells (BECs), which form the inner lining of the cerebrovasculature, are structurally and functionally distinct from endothelial cells in peripheral organs [5]. BECs form continuous, non-fenestrated capillaries with tight and adherens junctions that severely restrict paracellular diffusion across the BBB [1,6]. The permeability of the BBB is further reduced by limited fluid-phase transcytosis and active efflux transporters that eject unwanted substances from BECs [6]. The luminal side of BECs is lined by the glycocalyx, a negatively charged layer formed by glycans and glycoconjugates, which further supports BBB properties [4,7,8]. BBB integrity is an important factor for brain health, and its disruption is implicated in the pathogenesis of several neurodegenerative diseases, including Alzheimer’s disease and multiple sclerosis [2]. BBB dysfunction involves a range of pathological alterations, including increased inflammation, pericyte loss, oxidative stress, and alterations in protein and glycocalyx composition [2,4].
Detailed molecular and cellular characterization of brain microvessels is crucial for our understanding of BBB physiology and dysfunction in disease states. Here, we present a robust and reproducible protocol for microvessel isolation that utilizes mechanical dissociation and density gradient-based separation of parenchymal and vascular cells for subsequent microvessel analyses. Our protocol builds on previous methods [9,10] and enables a multitude of downstream analyses, including RNA sequencing (RNA-seq), proteomics, fluorescence imaging of cell surface and intracellular markers, western blotting, and ELISA. In Shi et al. (2025) [4], we utilized this protocol to interrogate molecular changes in the brain vasculature in aging and neurodegenerative disease, including alterations in cell surface glycosylation, reactive oxygen species (ROS), and protein abundance. Unlike approaches that isolate individual endothelial cells using transgenic markers (e.g., Tie2-eGFP) [11] or antibody-based selection (e.g., CD31+ sorting) [12], our protocol enables the study of intact microvascular units, allowing for the investigation of intercellular interactions and composite BBB properties. In addition, this method avoids harsh enzymatic dissociation or overly aggressive mechanical methods to isolate microvessels [13,14], which can result in molecular changes to the analyzed tissue [9]. This protocol has been successfully applied to fresh and frozen tissue of both murine and human origin.
Materials and reagents
Biological materials
1. C57BL/6J mice (Jackson Laboratory, catalog number: 000664)
Reagents
1. Bovine serum albumin (BSA) (Thermo Fisher Scientific, catalog number: 50-253-900)
2. Phosphate buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010049)
3. cOmpleteTM, EDTA-free protease inhibitor (PI) cocktail (Thermo Fisher Scientific, Roche, catalog number: 11873580001)
4. HBSS, calcium, magnesium, no phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 14025092)
5. Dextran from Leuconostoc mesenteroides, average molecular weight 60,000–75,000 g/mol (Millipore Sigma, Sigma-Aldrich, catalog number: D8821-100G)
6. 32% paraformaldehyde (formaldehyde) aqueous solution (PFA) (Thermo Fisher Scientific, Electron Microscopy Sciences, catalog number: 50-980-495)
7. Poly-D-Lysine (PDL) (Thermo Fisher Scientific, GibcoTM, catalog number: A3890401)
8. TRIS-buffered saline (TBS), 10×, pH 7.4 (Thermo Fisher Scientific, Thermo Scientific Chemical, catalog number: J60764.K2)
9. TWEEN® 20 (Millipore Sigma, Sigma-Aldrich, catalog number: P1379-100ML)
10. Normal donkey serum (Jackson ImmunoResearch, catalog number: 017-000-121)
11. TritonTM X-100 solution (TX-100) (Millipore Sigma, catalog number: 93443)
12. ProLongTM gold antifade mountant (Thermo Fisher Scientific, InvitrogenTM, catalog number: P36934); alternatively, VECTASHIELD HardSetTM antifade mounting medium with DAPI, 10 mL (Thermo Fisher Scientific, Vector Laboratories, catalog number: NC9029229)
Solutions
1. 1% BSA in PBS (see Recipes)
2. 1% BSA + 1× protease inhibitor (PI) in PBS (see Recipes)
3. 32% Dextran solution (see Recipes)
4. TBS-T (see Recipes)
5. 4% PFA solution (see Recipes)
6. Blocking solution (see Recipes)
Recipes
1. 1% BSA in PBS
Prepare in advance. Add 5 g of BSA to a sterile 500 mL bottle with 1× PBS and shake to mix. BSA will dissolve into PBS overnight. Store at 2–8 °C for up to four weeks.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 1% (w/v) | 5 g |
| PBS | 1× | 500 mL |
| Total | n/a | 500 mL |
2. 1% BSA + 1× protease inhibitor (PI) in PBS
Prepare and aliquot 25× PI according to the manufacturer’s instructions. Thaw a vial of 25× PI and add 200 μL of 25× PI to 4,800 μL of 1% BSA in PBS. Prepare 5 mL per sample. Store on ice and make fresh before every microvessel isolation.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 25× protease inhibitor | 1× | 200 μL |
| 1% BSA in PBS (from Recipe 1) | n/a | 4,800 μL |
| Total | n/a | 5,000 μL |
3. 32% Dextran solution
Prepare in advance. Add HBSS to 100 g of Dextran from Leuconostoc mesenteroides for a final volume of 312.5 mL. Add a clean magnetic stirring rod and stir at room temperature until dissolved. Can be stored at 4 °C for up to 3 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Dextran | 32% (w/v) | 100 g |
| HBSS | n/a | Fill to 312.5 mL |
| Total | n/a | 312.5 mL |
4. TBS-T
Dilute 100 mL of 10× TBS in 899.5 mL of ultrapure water and add 500 μL of Tween 20. Invert until completely mixed. Store for up to one year at room temperature.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tween 20 | 0.05% | 0.5 mL |
| 10× TBS | 1× | 100 mL |
| Ultrapure H2O | n/a | 899.5 mL |
| Total | n/a | 1,000 mL |
5. 4% PFA solution
Dilute 3.75 mL of 32% PFA in 26.25 mL of 1× PBS at room temperature. Make fresh before use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 32% PFA | 4% | 3.75 mL |
| PBS | n/a | 26.25 mL |
| Total | n/a | 30 mL |
6. Blocking solution
Add 3% normal donkey serum (NDS) and 0.3% TX-100 to TBS-T. Make enough for blocking and primary and secondary antibody staining steps. Calculate approximately 200 μL total per sample (volume will vary depending on the size of the hydrophobic rectangle). Make fresh before use.
Note: TBS-T can be replaced with TBS or PBS if sample permeabilization is not needed.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NDS | 3% | 45 μL |
| TX-100 | 0.3% | 4.5 μL |
| TBS-T (Recipe 4) | n/a | 1,450.5 μL |
| Total | n/a | 1,500 μL |
Laboratory supplies
1. Corning® 15 mL centrifuge tubes (Millipore Sigma, catalog number: CLS430790-500EA)
2. Corning® 50 mL centrifuge tubes (Millipore Sigma, catalog number: CLS4558-300EA)
3. VWR® disposable Petri dishes, 60 × 15, mono Petri dishes, fully stackable (EO sterilization) (VWR International, catalog number: 25384-168)
4. Corning® tissue culture–treated culture dishes, 150 mm × 25 mm (Corning, Millipore Sigma, catalog number: CLS430599-60EA)
5. VWR® razor blades (VWR International, catalog number: 55411-050)
6. Corning® cell strainer, pore size 40 μm (Corning, Millipore Sigma, catalog number: CLS431750-50EA)
7. Kimberly-Clark ProfessionalTM Kimtech ScienceTM KimwipesTM delicate task wipers, 1 ply (Kimberly-Clark Professional, Thermo Fisher Scientific, catalog number: 06-666)
8. Snap-cap low-retention microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 3448PK)
9. FisherbrandTM SuperfrostTM Plus microscope slides (Thermo Fisher Scientific, catalog number: 12-550-15)
10. ImmEdge® hydrophobic barrier PAP pen (Vector Laboratories, catalog number: H-4000)
11. FisherbrandTM Premium cover glasses (Thermo Fisher Scientific, catalog number: 12-548-5A)
12. FisherbrandTM aluminum foil, standard-gauge roll (Thermo Fisher Scientific, catalog number: 01-213-102)
13. TipOne® filter tips, 1,000 μL graduated (USA Scientific, catalog number: 1125-7810)
14. TipOne® filter tips, 200 μL graduated (USA Scientific, catalog number: 1120-8810)
15. NuncTM serological pipettes, 5 mL (Thermo Fisher Scientific, catalog number: 170355N)
16. NuncTM serological pipettes, 10 mL (Thermo Fisher Scientific, catalog number: 170356N)
17. NuncTM serological pipettes, 25 mL (Thermo Fisher Scientific, catalog number: 170357N)
Equipment
1. Wheaton® 357424 glass 7 mL Tenbroeck tissue grinder set, grinding chamber O.D. × L: 16 × 82 mm (case of 2) (Dounce homogenizer) [DWK Life Sciences Inc. (Wheaton), catalog number: 357424]
2. CAPP motorized pipette controller, 0.1–100 mL (Pipette.com, catalog number: PA-100)
3. Centrifuge 5810/5810 R (Eppendorf, catalog number: 022625004)
4. CorningTM LSETM low-speed orbital shaker (Corning, Thermo Fisher Scientific, catalog number: 10-320-813)
5. NalgeneTM polycarbonate Erlenmeyer flasks (Thermo Fisher Scientific, catalog number: 4103-0250)
6. Confocal laser-scanning microscope (Zeiss, catalog number: LSM880)
Software and datasets
1. ZEN Microscopy Software (Carl Zeiss Microscopy GmbH, Version 3.079.0006)
2. ImageJ (National Institutes of Health and the Laboratory for Optical and Computational Instrumentation, Version 1.54p or higher)
3. GraphPad Prism (Graphpad Software, Inc., Version 10.4.1 or higher)
Procedure
文章信息
稿件历史记录
提交日期: May 29, 2025
接收日期: Jul 2, 2025
在线发布日期: Jul 22, 2025
出版日期: Aug 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Buff, J. K., Bertozzi, C. R., Wyss-Coray, T. and Shi, S. M. (2025). Isolation and Imaging of Microvessels From Brain Tissue. Bio-protocol 15(15): e5410. DOI: 10.21769/BioProtoc.5410.
分类
神经科学 > 神经系统疾病 > 血脑屏障
细胞生物学 > 组织分析 > 组织分离
神经科学 > 神经系统疾病 > 神经退行性病变
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












