- EN - English
- CN - 中文
Reprogramming White Fat Cells for Adipose Manipulation Transplantation (AMT) Therapy
通过重编程白色脂肪细胞实现脂肪操控移植(AMT)治疗
(*contributed equally to this work) 发布: 2025年08月05日第15卷第15期 DOI: 10.21769/BioProtoc.5405 浏览次数: 2223
评审: Andrea GramaticaAnonymous reviewer(s)

相关实验方案
利用EpiCRISPR系统通过靶向DNA甲基化诱导Alpha TC1-6细胞产生胰岛素
Marija B. Đorđević [...] Melita S. Vidaković
2025年10月20日 1215 阅读
Abstract
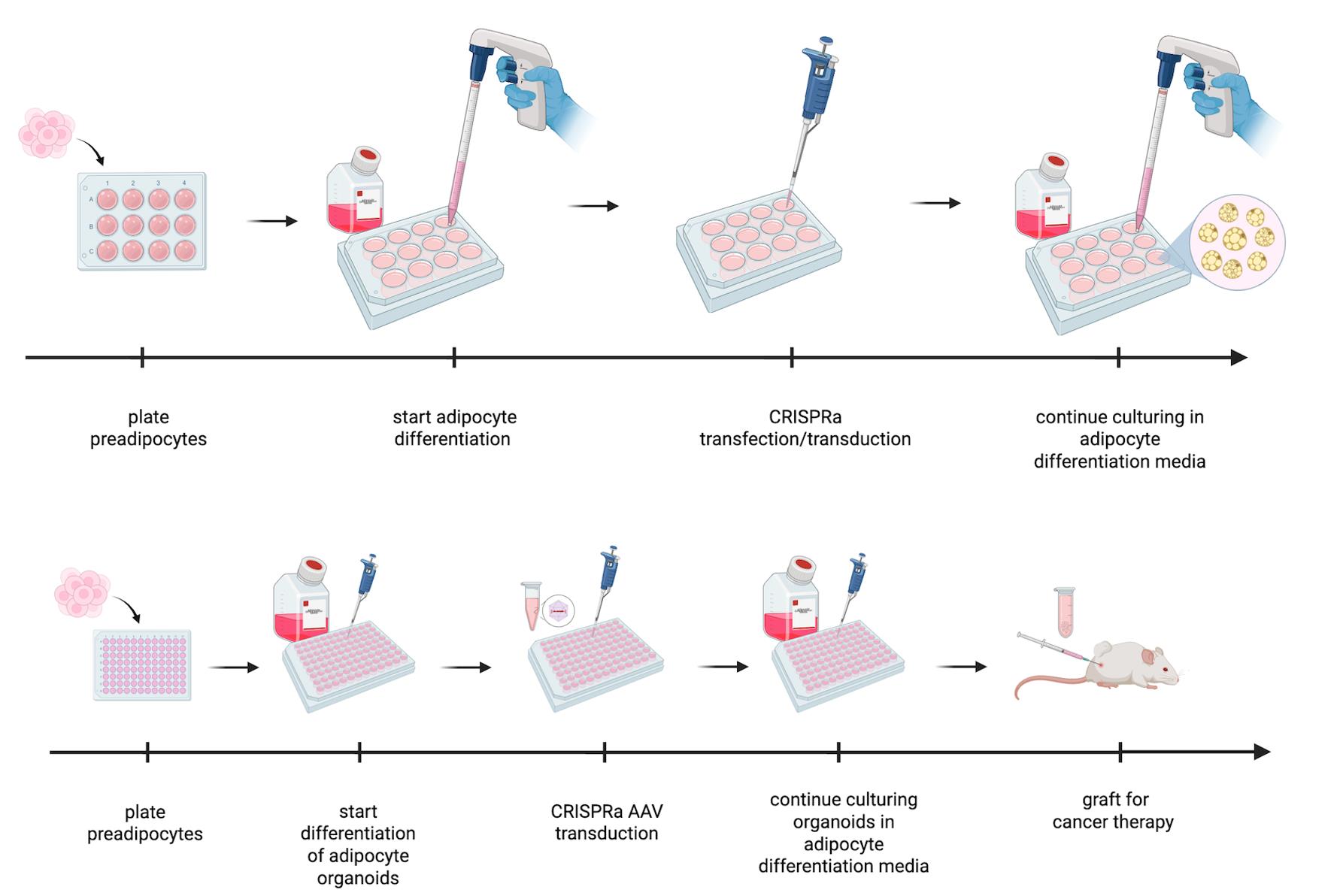
Adipocytes are endocrine cells that function as the main energy storage in our body. They are commonly used in clinical procedures, including their removal via liposuction and transplantation in plastic surgery. Building on this, adipocytes can be used for ex vivo cellular manipulations, enabling therapeutic modifications that can provide beneficial clinical outcomes after transplantation. Here, we provide a detailed protocol on how to modify adipocytes and adipose organoids using CRISPR activation (CRISPRa), a technology termed adipose manipulation transplantation (AMT).
Key features
• This protocol is used to generate adipocytes and adipose organoids from human preadipocytes that can be genetically engineered for therapeutic purposes, including cancer metabolic therapy.
Keywords: Adipocytes (脂肪细胞)Graphical overview
Background
White adipose tissue (WAT) is a major energy storage organ and endocrine tissue. It primarily stores triglycerides and secretes adipokines, such as leptin, adiponectin, and other lipids that regulate various biological functions, including appetite, metabolism, and insulin homeostasis [1]. In contrast, brown adipose tissue (BAT) dissipates energy by non-shivering thermogenesis to maintain body temperature and was shown to be stimulated in adults upon cold exposure and to be inversely correlated with adiposity [2–6]. BAT not only has a high capacity for glucose and fatty acid (FA) uptake, but also secretes adipokines, all of which contribute to insulin sensitivity [7–9].
Subcutaneous WAT is capable of changing into a BAT-like tissue, called beiging [5,6]. Like brown adipocytes, beige adipocytes can convert energy to heat and contribute to whole-body energy expenditure. As ~90% of stored fat is subcutaneous in humans, inducing beiging by reprogramming WAT could be an effective strategy to improve metabolic outcomes. This is controlled by the upregulation of essential transcriptional regulators and enzymes, such as the uncoupling protein 1 (UCP1). In BAT, thermogenesis is mainly dependent on UCP1 to “convert” substrates to heat; overexpression of this gene in mice leads to reduced WAT and body weight and increased recruitment of brown-like fat cells within WAT [10].
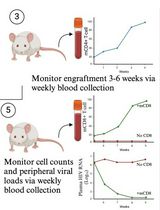
Liposuction and fat transplantation are commonly used in many surgical procedures, such as aesthetic and reconstructive surgery. Due to successful engraftment, adipose tissue transplantation could also be used for therapeutic treatments [11]. Several reports using rodent models have shown that BAT transplantation has beneficial metabolic outcomes [7,12–14]. In humans, there is a growing interest to carry out human adipose tissue grafting by using adipose organoids, as they have several advantages over WAT: 1) they can recapitulate the heterogeneity of tissue of origin, 2) they respond to endogenous stimuli, 3) they provide appropriate tissue microenvironments, and 4) they are more resistant to trauma and can provide long-term survival following transplantation [15–17].
Tumors are complex tissues composed of cancerous and non-cancerous cells in a hypoxic and nutrient-deprived microenvironment. The tumor microenvironment (TME) contains heterogeneous cell populations, including immune cells, mesenchymal support cells, and matrix components contributing to tumor growth and progression [18]. In order to survive this environment, tumors are capable of reprogramming metabolic pathways to better utilize available substrates in the surrounding TME [19]. During hypoxia, cancer cells also undergo metabolic reprogramming to increase lipid utilization [20,21]. Fatty acids (FAs) produce twice the energy of glucose. During periods of energy deprivation, WAT releases FAs into circulation by lipolysis. Cancer cells take up these FAs via the fatty acid transporter, CD36 [22]. Thus, WAT, the main source of circulating FAs, serves as an important energy source for cancer cells.
There are many efforts to target cancer glucose metabolism for therapeutic purposes (for a detailed review, see [19]). In addition, several drugs are also used to target lipid metabolism in cancer [23–26]. However, they have limited efficacy and off-target effects, and thus, very few of these drugs have made it into the clinic. CRISPR technology provides a novel way to modulate adipocytes and adipose stem cells to harness them for therapeutic purposes. We recently showed how these tools could be used to modify adipocytes or adipose organoids so that they suppress tumor progression [27], a technology termed adipose manipulation transplantation (AMT).
Here, we provide a detailed protocol on how to generate and manipulate adipocytes or adipose organoids for AMT using CRISPR activation (CRISPRa) with adeno-associated virus (AAV). We note that upregulation of genes could also be achieved via other modalities, such as zinc fingers, transcription activator-like effectors (TALEs), and cDNA expression vectors, and that other delivery methods could be used instead of AAV. In addition, while we mainly used UCP1 as our target gene to generate “beige” cells or organoids [27], other genes could be targeted similarly. This includes not only BAT-associated genes but also genes involved in glucose transport, glucose metabolism, fatty acid oxidation, cholesterol synthesis, and many other metabolic pathways to outcompete cancer. We also note that these cells and organoids are great secretors and could be modified to secrete molecules that target the TME. Finally, this protocol could also be used to modify these cells and organoids, such that they can be used for other metabolic diseases. In summary, we provide a detailed experimental protocol for adipocyte and adipose organoid generation from human preadipocytes and how to reprogram them with CRISPRa. This technology holds promise not only for cancer therapy but also for many additional metabolic diseases.
Materials and reagents
Biological materials
1. Human preadipocytes
2. Plasmid encoding guide RNA under the U6 promoter for the gene of interest; we used Addgene # 217015
3. Plasmid encoding a nuclease-deficient dCas9 fused with a transcriptional activator; we used Addgene # 115790
4. AAV9-encoding guide RNA under U6 promoter for gene of interest
5. AAV9-encoding dCas9 fused with a transcriptional activator
Reagents
1. DMEM, high glucose (Fisher Scientific, catalog number: 11-965-118)
2. Heat-inactivated FBS (Fisher Scientific, catalog number: 10438026)
3. Penicillin-streptomycin (Fisher Scientific, catalog number: 15140122)
4. Trypsin-EDTA 0.25% (Fisher Scientific, catalog number: 25200056)
5. DPBS, no calcium, no magnesium (Fisher, catalog number: 14190144)
6. Isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, catalog number: 410957)
7. Dexamethasone (Sigma-Aldrich, catalog number: D1756)
8. Insulin (Sigma-Aldrich, catalog number: I9278)
9. X-tremeGENETM HP DNA transfection reagent (Roche, catalog number: 6366236001)
10. Opti-MEMTM I reduced serum medium (Gibco, catalog number: 31985062)
11. CombimagTM transfection reagent (Oz Biosciences, catalog number: CM21000)
12. DMEM, high glucose, pyruvate (Fisher Scientific, catalog number: 11-995-065)
13. TransIT®-293 transfection reagent (Mirus, catalog number: MIR 2706)
14. AAVpro® Purification kit maxi/midi (all serotypes) (Takara Bio, catalog number: 6666/6675)
15. AAVpro® Titration kit (for real-time PCR) v. 2 (Takara Bio, catalog number: 6233)
16. KOD OneTM PCR master mix (Diagnocine, catalog number: TYB-KMM-201)
17. BstXI (Fisher Scientific, catalog number: FD1024); specific to this plasmid, but can be different for others
18. XhoI (Fisher Scientific, catalog number: FD0694); specific to this plasmid, but can be different for others
19. 10× FastDigest green buffer (Fisher Scientific, catalog number: FD1024)
20. QIAquick Gel Extraction kit (Qiagen, catalog number: 28704)
21. NEBuilder® HiFi DNA Assembly master mix (NEB, catalog number: E2621)
22. SeaKem® LE agarose (Lonza, catalog number: 50004)
23. StellarTM competent cells (Takara Bio, catalog number: 636766)
24. Luria Broth base (Miller’s LB broth base)TM, powder (Fisher, catalog number: 12795084)
24. LB agar plates with ampicillin, 100 mm (Teknova, catalog number: L1004)
25. Ampicillin, sodium salt powder (Corning, catalog number: 61-238-RM)
26. QiaPrep Spin MiniPrep (Qiagen, catalog number: 27104)
27. TRIzol reagent (Fisher Scientific, catalog number: 15596026)
28. Chloroform (Sigma-Aldrich, catalog number: 472476)
29. RNeasy Mini kit (Qiagen, catalog number: 74104)
30. qScript cDNA Synthesis kit (Quantabio, catalog number: 95047)
31. SsoFast EvaGreen Supermix (Bio-Rad, catalog number: 1725205)
32. Ultrapure DNase/RNase-free distilled water (nuclease-free water) (Fisher Scientific, catalog number: 10-977-023)
33. Clorox germicidal bleach (Clorox, catalog number: 30966)
Solutions
1. sgRNA PCR mix (see Recipes)
2. AAV backbone digestion mix (see Recipes)
3. NEBuilder® HiFi DNA assembly reaction (see Recipes)
4. LB media (see Recipes)
5. Preadipocyte culturing medium (see Recipes)
6. Preadipocyte transfection mix (see Recipes)
7. Adipocyte differentiation medium (see Recipes)
8. Transfection mix (see Recipes)
9. Reverse transcription mix (see Recipes)
10. qPCR mix (see Recipes)
11. AAVproHEK293T culture medium (see Recipes)
12. AAV transfection mix (see Recipes)
13. AAV media change medium (see Recipes)
Recipes
1. sgRNA PCR mix
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10 μM forward primer | 0.5 μM | 1.5 μL |
| 10 μM reverse primer | 0.5 μM | 1.5 μL |
| KOD One | 1× | 12.5 μL |
| Template (a sgRNA plasmid) | <250 ng | variable |
| Nuclease-free water | up to 25 μL | |
| Total | 25 μL |
2. AAV backbone digestion mix
| Reagent | Quantity or Volume |
|---|---|
| AAV backbone | 1 μg |
| BstXI | 1 μL |
| XhoI | 1 μL |
| 10× FastDigest green buffer | 5 μL |
| Nuclease-free water | 42 μL |
| Total | 50 μL |
3. NEBuilder® HiFi DNA assembly reaction
| Reagent | Quantity or Volume |
|---|---|
| Digested plasmid | 50 ng |
| Insert | 10 ng |
| NEBuilder® HiFi DNA assembly master mix | 10 μL |
| Nuclease-free water | up to 10 μL |
| Total | 20 μL |
4. LB media
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Luria Broth base | 25 g/L | 25 g |
| Distilled water | 1 L | |
| Total | 1 L |
5. Preadipocyte culturing medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM, high glucose | 89% | 445 mL |
| Heat-inactivated FBS | 10% | 50 mL |
| Penicillin-streptomycin | 1% | 5 mL |
| Total | 500 mL |
6. Preadipocyte transfection mix (per well of a 12-well plate)
| Reagent | Quantity or Volume |
|---|---|
| Opti-MEM | 100 μL |
| dCas9 plasmid | 1 μg |
| gRNA plasmid | 1 μg |
| X-tremeGENE reagent | 2 μL |
| Total | ~102 μL |
7. Adipocyte differentiation medium
| Reagent | Final concentration | Quantity or Volume (for 10 mL) |
|---|---|---|
| DMEM, high glucose | 90% | 9 mL |
| Heat-inactivated FBS | 10% | 1 mL |
| IBMX | 0.5 mM | 10 μL (of 0.5 M stock) |
| Dexamethasone | 1 μM | 10 μL (of 1 mM stock) |
| Insulin | 10 μg/mL | 10 μL (of 10 mg/mL stock) |
| Total | ~10 mL |
8. Transfection mix (per well of a 12-well plate)
| Reagent | Quantity or Volume |
|---|---|
| Opti-MEM | 100 μL |
| dCas9 plasmid | 1 μg |
| gRNA plasmid | 1 μg |
| X-tremeGENE reagent | 2 μL |
| Combimag reagent | 2 μL |
| Total | ~104 μL |
9. Reverse transcription mix (per 1 sample)
| Reagent | Volume |
|---|---|
| Nuclease-free water | 22 μL |
| qScript reaction mix (5×) | 8 μL |
| qScript RT | 2 μL |
| Total | 32 μL |
10. qPCR mix (per 1 sample)
| Reagent | Volume |
|---|---|
| SsoFast EvaGreen supermix | 5 μL |
| Forward primer (final concentration 300–500 nM) | 0.25 μL |
| Reverse primer (final concentration 300–500 nM) | 0.25 μL |
| Nuclease-free water | 1.5 μL |
| Total | 7 μL |
11. AAVproHEK293T culture medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM, high glucose, pyruvate | 89% | 445 mL |
| Heat-inactivated FBS | 10% | 50 mL |
| Penicillin-streptomycin | 1% | 5 mL |
| Total | 500 mL |
12. AAV transfection mix (per one 150 mm dish)
| Reagent | Quantity or Volume |
|---|---|
| Opti-MEM | 4 mL |
| TransIT®-293 transfection reagent | 120 μL |
| Your own vector (either gRNA or dCas9-VP64) | 20 μg |
| pHelper vector | 20 μg |
| pRC9 (AA9 serotype) vector | 20 μg |
| Total |
13. AAV media change medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM, high glucose, pyruvate | 98% | 490 mL |
| Heat-inactivated FBS | 2% | 10 mL |
| Total | 500 mL |
Laboratory supplies
1. Nunclon Sphera-treated 96-well U-shaped-bottom microplate (Thermo Scientific, catalog number: 174925) (for adipose organoids)
2. Common cell culture supplies (cell culture plates/flasks/consumables, pipettes, pipet-aid, serological tips)
3. Common laboratory consumables (gloves, pipettes, pipette tips, centrifuge tubes)
Equipment
1. Water/bead bath (Thermo Fisher Scientific, model: TSGP05)
2. Tissue culture incubator (37 °C, 5% CO2) (Thermo Fisher Scientific, model: 3110)
3. Tissue culture hood (Thermo Fisher Scientific, model: 1300 Series A2 Biosafety Cabinet)
4. Magnetic plate for magnetofection (Oz Biosciences, model: MF10000)
5. Centrifuge with refrigeration that can spin 15 or 50 mL conical tubes (Beckman Coulter, model: B08708)
6. Microcentrifuge with refrigeration capability (Eppendorf, model: 5417R)
7. Vortex mixer (Fisher Scientific, model: Vortex-Genie 2 Mixer)
8. Heat block (Fisher Scientific, model: 88-870-001)
9. PCR machine (ProFlex PCR System, Fisher Scientific, model: 4484073)
10. Bacterial shaking incubator (New Brunswick, model: Excella E24)
11. Plate mixer (Fisher Scientific, model: 88-861-023)
12. NanoDrop 8000 (Fisher Scientific, model: ND-8000-GL)
13. qPCR machine (Thermo Fisher Scientific, model: QuantStudio 6)
Procedure
文章信息
稿件历史记录
提交日期: May 21, 2025
接收日期: Jul 1, 2025
在线发布日期: Jul 22, 2025
出版日期: Aug 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
An, K., Ito, Y. and Ahituv, N. (2025). Reprogramming White Fat Cells for Adipose Manipulation Transplantation (AMT) Therapy. Bio-protocol 15(15): e5405. DOI: 10.21769/BioProtoc.5405.
分类
细胞生物学 > 细胞工程 > CRISPR-cas9
细胞生物学 > 细胞移植 > 异种移植
干细胞 > 成体干细胞 > 脂肪干细胞
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link