- EN - English
- CN - 中文
Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
活体叶片切片成像观察细胞内叶绿体运动及细胞间相互作用
(§Technical contact: yuta.kato2145@gmail.com) 发布: 2025年08月05日第15卷第15期 DOI: 10.21769/BioProtoc.5404 浏览次数: 2277
评审: Samik BhattacharyaAnonymous reviewer(s)
Abstract
In response to environmental changes, chloroplasts, the cellular organelles responsible for photosynthesis, undergo intracellular repositioning, a phenomenon known as chloroplast movement. Observing chloroplast movement within leaf tissues remains technically challenging in leaves consisting of multiple cell layers, where light scattering and absorption hinder deep tissue visualization. This limitation has been particularly problematic when analyzing chloroplast movement in the mesophyll cells of C4 plants, which possess two distinct types of concentrically arranged photosynthetic cells. In response to stress stimuli, mesophyll chloroplasts aggregate toward the inner bundle sheath cells. However, conventional methods have not been able to observe these chloroplast dynamics over time in living cells, making it difficult to assess the influence of adjacent bundle sheath cells on this movement. Here, we present a protocol for live leaf section imaging that enables long-term and detailed observation of chloroplast movement in internal leaf tissues without chemical fixation. In this method, a leaf blade section prepared either using a vibratome or by hand was placed in a groove made of a silicone rubber sheet attached to a glass slide for microscopic observation. This technique allows for the quantitative tracking of chloroplast movement relative to the surrounding cells. In addition, by adjusting the sectioning angle and thickness of the unfixed leaf sections, it is possible to selectively inactivate specific cell types based on their size and shape differences. This protocol enables the investigation of the intercellular interactions involved in chloroplast dynamics in leaf tissues.
Key features
• Thin leaf sections prepared while still alive enable prolonged microscopic observation of chloroplast movement within the leaf tissue.
• Selective cell inactivation can be achieved by adjusting the slice thickness and angle.
• This method is applicable to a wide range of plant species.
Keywords: C4 plant (碳四植物)
Graphical overview
Background
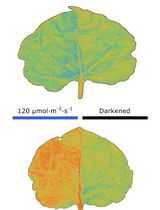
Chloroplasts are organelles responsible for photosynthesis in leaves that change their intracellular positions in response to various environmental stimuli. In C4 plants, which perform photosynthesis across two types of photosynthetic cells, mesophyll and bundle sheath cells, an aggregative movement of mesophyll chloroplasts has been observed. Under stress conditions such as high-intensity light, drought, and salinity, mesophyll chloroplasts aggregate to bundle sheath cells [1–3]. Techniques such as transmittance measurements, band assays, polarized light irradiation, and microbeam irradiation have been used to elucidate the mechanisms involved in chloroplast movement [4–6]. However, these conventional methods have been insufficient for investigating the mechanism of mesophyll chloroplast aggregative movement in C4 plants, particularly the potential involvement of adjacent bundle sheath cells. This is because C4 plants possess a concentric leaf structure composed of multiple cell layers, and mesophyll chloroplasts aggregate toward the inner bundle sheath cells. Such inward movements cannot be accurately measured using transmittance-based methods or similar techniques, and the behavior of individual mesophyll chloroplasts relative to adjacent bundle sheath cells within the tissue remains unclear. Although thin sectioning after chemical fixation allows for the precise observation of chloroplast positioning, biological activity ceases after fixation, preventing the assessment of physiological responses.
To address these challenges, we developed a live leaf-section imaging technique that enables the long-term observation of chloroplast movement in unfixed leaf sections. This technique requires a certain level of technical proficiency; however, it is fundamentally simple because it involves thin sectioning live tissues without chemical fixation. This allows for quantitative analysis of chloroplast movement relative to bundle sheath cells, providing insights into intercellular interactions and movement dynamics. Furthermore, by adjusting the sectioning angle and thickness of the unfixed leaf sections, we successfully prepared sections that excluded the contents of bundle sheath cells, enabling investigation of the role of adjacent bundle sheath cells in chloroplast movement. Using these approaches, we demonstrated that mesophyll chloroplast movement in C4 plants is an intercellular response [7].
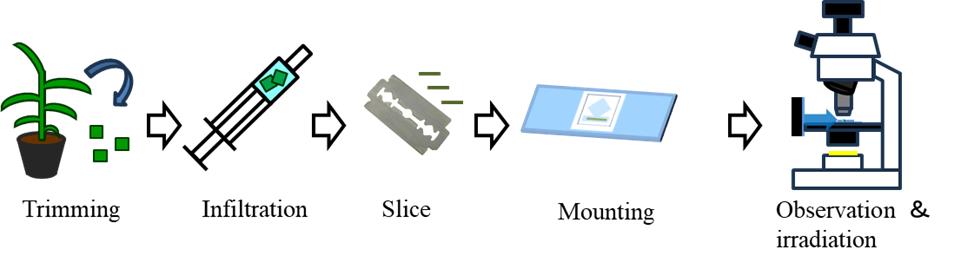
In this protocol, using the C4 plant Eleusine coracana (finger millet) as an example, we describe the preparation of leaf sections, the setup for observation, and the analytical methods used to track chloroplast movement. Additionally, we introduce a hand-sectioning method that allows for the preparation of unfixed sections in plant species that are difficult to section using a vibrating microtome owing to leaf hardness. Our method enables the observation of entire leaf tissues in a living state, making it applicable to the study of chloroplast movement and providing a valuable tool for analyzing tissue-level physiological responses.
Materials and reagents
Biological materials
1. Eleusine coracana (finger millet), seed purchased from Snow Brand Seed, Japan
2. Arabidopsis thaliana, accession Col-0
Reagents
1. Agar powder for plant culture medium (FUJIFILM Wako Pure Chemical, Japan, catalog number: 016-11875)
Solutions
1. Agar solution for embedding (see Recipes)
2. Agar solution for mounting (see Recipes)
Recipes
1. Agar solution for embedding
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Agar powder | 5% w/v | 10 g |
| Purified water | n/a | Up to 200 mL |
Melt the solution in a microwave before use. It can be stored in the solidified state under refrigerated conditions.
Caution: Continuously monitor the agar during heating to prevent spillage. Stirring after prolonged heating may cause sudden boiling; therefore, heating and stirring must be performed intermittently.
2. Agar solution for mounting
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Agar powder | 0.1% w/v | 0.1 g |
| Purified water | n/a | Up to 100 mL |
Melt the solution in a microwave before use. It does not completely solidify even after cooling. To ensure uniform viscosity, the mixture should be stirred while cooling. Reuse is not possible.
Laboratory supplies
1. Silicon rubber plate, 100 × 100 mm, 3 mm thick (Ohsato, Japan, catalog number: 901-1418)
2. Razor blade (FEATHER, Japan, catalog number: FH-10B)
3. 10 mL syringe (Terumo, Japan, catalog number: SS-10SZ)
4. 300 mL flask (SIBATA, Japan, catalog number: 010530-300A)
5. Small glass Petri dish (AS ONE, Japan, catalog number: 1-4564-01)
6. Micro spatula (AS ONE, Japan, catalog number: 6-524-01)
7. Instant adhesive (Toagosei, Japan, catalog number: 12470)
8. Tweezers (Rubis, Switzerland, catalog number: 5-SA)
9. Inoculation loop, ϕ 2 mm (SCI, SCIENCE CENTER, USA, catalog number: 3512)
10. Ultra-transparent silicone rubber film, thickness 200 μm (AS ONE, Japan, catalog number: 3-9207-06)
11. Slide glass (Matsunami Glass, Japan, catalog number: S1214)
12. Cover glass, 18 × 18 mm, thickness 0.13–0.17 mm (No. 1) (Matsunami Glass, Japan, catalog number: C218181)
13. Paper wiper (Kimwipes) (Nippon Paper Crecia, Japan, catalog number: 62011)
Equipment
1. Microwave (e.g., Panasonic, Japan, model: NE-EG-211)
2. Vibratome (e.g., Leica Microsystems, Germany, model: VT1200S; Dosaka EM, Japan, model: DTK 300 W)
3. Microscope (e.g., Olympus, Japan, model: BX51 equipped with fluorescence illuminator BX-URA2)
4. CMOS camera (e.g., Olympus, Japan, model: DP74)
5. Red filter on the microscope light (e.g., Kenko, Japan, catalog number: 000000003411, https://shop.kenko-tokina.co.jp/view/item/000000003411)
6. Red filter in the light pass of the microscope (Edmund, USA, catalog number: 15-226)
7. Blue light LED panel (CCS, Japan, catalog number: ISL-150X150-BB45)
8. Light meter (LI-COR, USA, model: LI-250A)
9. Light table (e.g., Shosai, Japan, model: A4-LED Tracing Stand, Amazon ASIN: B084M65BB3)
Software and datasets
1. Microscope imaging software (e.g., Olympus, cellSense, or Advan Vision, AdvanView)
2. Fiji (ImageJ) software (Version 1.54m, https://imagej.net/software/fiji/)
Procedure
文章信息
稿件历史记录
提交日期: Apr 18, 2025
接收日期: Jun 30, 2025
在线发布日期: Jul 22, 2025
出版日期: Aug 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Kato, Y., Oi, T., Sato, Y. and Taniguchi, M. (2025). Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions. Bio-protocol 15(15): e5404. DOI: 10.21769/BioProtoc.5404.
分类
植物科学 > 植物细胞生物学 > 细胞成像
细胞生物学 > 细胞成像 > 活细胞成像
植物科学 > 植物细胞生物学 > 细胞结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link