- EN - English
- CN - 中文
Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L
基于荧光偏振的组织蛋白酶L抑制剂高通量筛选方法
(*contributed equally to this work) 发布: 2025年07月20日第15卷第14期 DOI: 10.21769/BioProtoc.5400 浏览次数: 1256
评审: Neha NandwaniAnonymous reviewer(s)
Abstract
Cathepsin L (CTSL), a lysosomal cysteine protease belonging to the papain-like protease family, is primarily involved in intracellular protein degradation, antigen processing, and extracellular matrix remodeling. It plays critical roles in pathological conditions, including cancer metastasis, neurodegenerative disorders, and viral infection, due to dysregulated activity or overexpression. Thus, inhibitors targeting CTSL are under investigation for therapeutic applications. Current approaches for identifying CTSL inhibitors predominantly rely on fluorescence-labeled substrates, fluorescence resonance energy transfer (FRET), and cell-based screening assays. Here, we applied the principle of fluorescence polarization (FP) to the detection of substrate cleavage activity by CTSL through changes in millipolarization unit (mp) values and established a cost-effective, quantitative, reagent- and time-saving inhibitor high-throughput screening (HTS) assay. We also provide detailed steps for the expression and purification of highly active CTSL from eukaryotic cells, which lays a solid foundation for the FP-based assay. A key advantage of this assay lies in its reduced susceptibility to fluorescence interference, as the fluorescein isothiocyanate (FITC) fluorophore exhibits high quantum efficiency with an emission peak at 535 nm—a wavelength range distinct from most naturally occurring fluorescent molecules. The assay’s adaptability to reaction time, temperature, and dimethyl sulfoxide (DMSO) concentration minimizes false-positive or false-negative results caused by minor experimental inconsistencies, streamlining the screening process. Furthermore, the protocol requires fewer operational steps, reduced incubation time, and lower quantities of CTSL and substrates compared to conventional methods. This rapid, cost-effective, and scalable approach aligns well with the demands of HTS platforms.
Key features
• Detailed procedures for eukaryotic expression, purification, and identification of active recombinant CTSL and determination of its biological activity.
• Full description for application of fluorescence polarization (FP)-based high-throughput screening (HTS) assay targeting CTSL.
• Elaboration for the application of the FP-based assay in CTSL activity evaluation or drug discovery.
• Protocol is readily adaptable to other proteases with a similar catalytic mechanism.
Keywords: Cathepsin L (组织蛋白酶L)Graphical overview
Background
Being an endosomal cysteine protease, cathepsin L (CTSL) serves as a critical mediator of viral entry by proteolytically activating fusion proteins essential for host cell membrane penetration. This mechanism has been implicated in diverse viral families, including caliciviruses, Ebola virus, reovirus, coronaviruses, and hepatitis E virus [1]. Given that viral entry constitutes the initial step of infection and replication, CTSL inhibition represents a promising therapeutic strategy to disrupt viral lifecycles. Beyond virology, CTSL exhibits significant oncogenic potential through its capacity to remodel the extracellular matrix (ECM). By degrading structural ECM components and basement membranes, CTSL facilitates tumor cell migration, angiogenesis, and metastasis [2]. Notably, its overexpression in esophageal squamous cell carcinoma (ESCC) correlates with tumor-associated macrophage activity, linking protease function to immune and inflammatory pathways [3]. These multifaceted roles in disease pathogenesis underscore the urgent need for targeted CTSL inhibitors.
Current CTSL inhibitor screening predominantly employs fluorescence-based assays (e.g., FRET, fluorescence correlation spectroscopy) and cell-based models, yet each method presents inherent constraints. FRET assays require precise spectral compatibility between donor-acceptor pairs to minimize crosstalk, necessitating extensive optimization of fluorophore combinations [4]. Fluorescence correlation spectroscopy suffers from photobleaching artifacts and limited throughput [5], while cell-based approaches are time-intensive. These challenges highlight the demand for robust, high-efficiency screening alternatives.
First conceptualized in 1926, fluorescence polarization (FP) technology exploits rotational dynamics of fluorescent molecules under polarized excitation [6]. Crucially, FP eliminates separation steps by performing homogeneous solution-phase measurements, offering advantages in speed, sensitivity, and operational simplicity [7]. When a fluorophore-labeled ligand remains unbound, rapid Brownian motion (nanosecond-scale rotation) induces significant emission depolarization, yielding low millipolarization (mP) values. Ligand binding to macromolecular targets (e.g., avidin) restricts rotational freedom, preserving polarization and generating elevated mP signals. Key merits of FP include high sensitivity, miniaturization compatibility, minimal sample volumes, operational efficiency, and low interference. These attributes position FP as a versatile tool for high-throughput drug discovery pipelines.
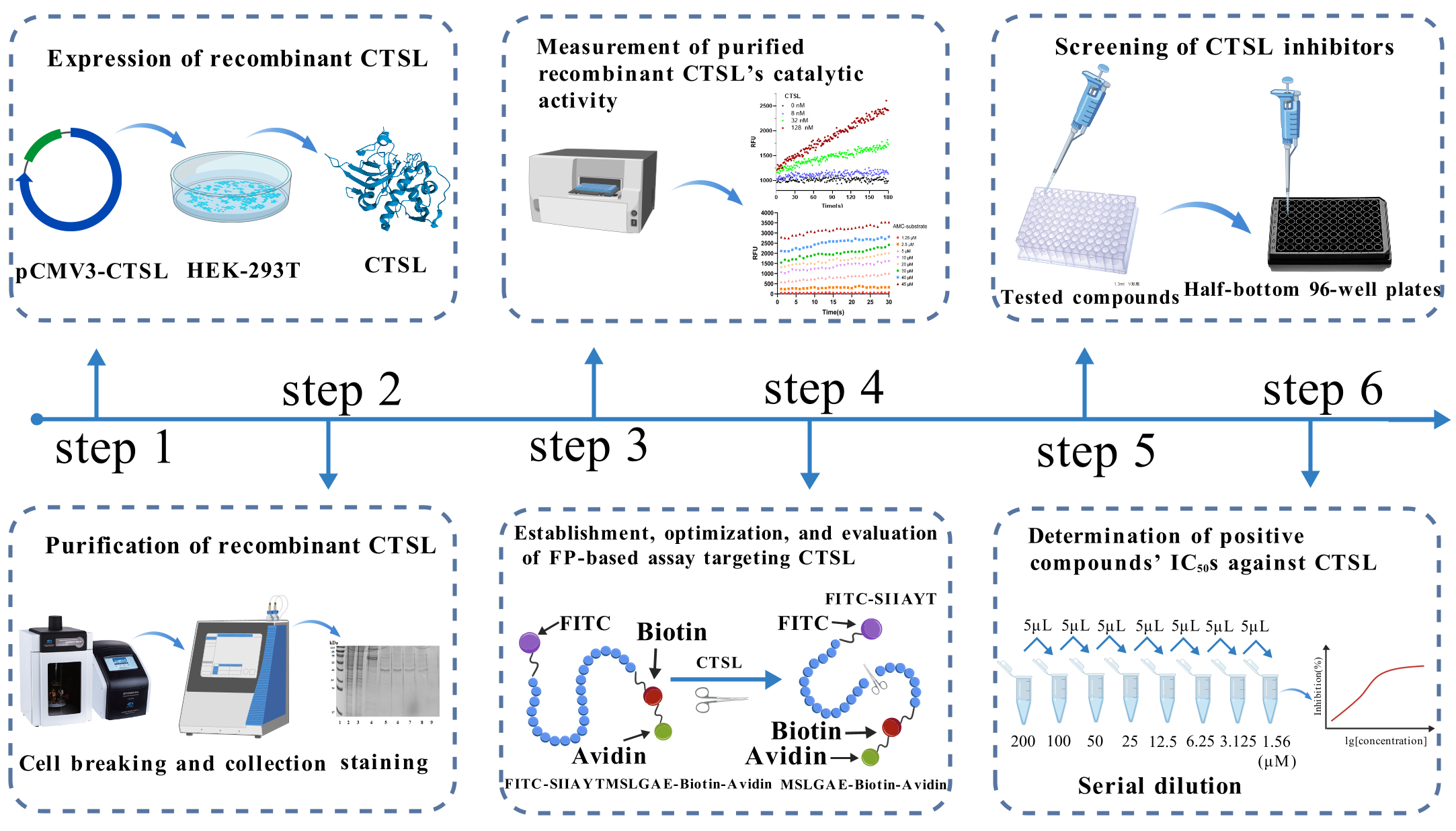
Based on this principle, we established a high-throughput screening (HTS) model for CTSL inhibitors. Our assay utilizes a bifunctional substrate (SIIAYTMSLGAE) with FITC conjugation at the C-terminus and biotin at the N-terminus. In uninhibited conditions, CTSL cleaves the substrate into two fragments: FITC-SIIAYT (low molecular weight, rapid rotation, low mP) and MSLGAE-Biotin. Conversely, CTSL inhibition preserves substrate integrity, and avidin-mediated biotin capture further amplifies mass differences for enhanced detection by generating a high-mass FITC-SIIAYTMSLGAE-Biotin complex with restricted rotation, producing elevated mP signals proportional to inhibitor potency (Figure 1) [8]. This FP-based system enables rapid, separation-free quantification of inhibitory activity through mP value modulation. To establish this HTS model, we first expressed CTSL in a eukaryotic system and purified the recombinant protein. The enzymatic activity of purified CTSL was then assessed using the fluorogenic substrate Z-Phe-Arg-AMC, as previously described [9]. CTSL specifically cleaves the amide bond between arginine (Arg) and 7-amino-4-methylcoumarin (AMC) in Z-Phe-Arg-AMC, releasing free AMC, which exhibits strong fluorescence emission at 440 nm upon excitation at 365 nm, while the intact substrate (Z-Phe-Arg-AMC) is non-fluorescent. The rate of AMC release is directly proportional to CTSL enzymatic activity, allowing for real-time kinetic measurements.
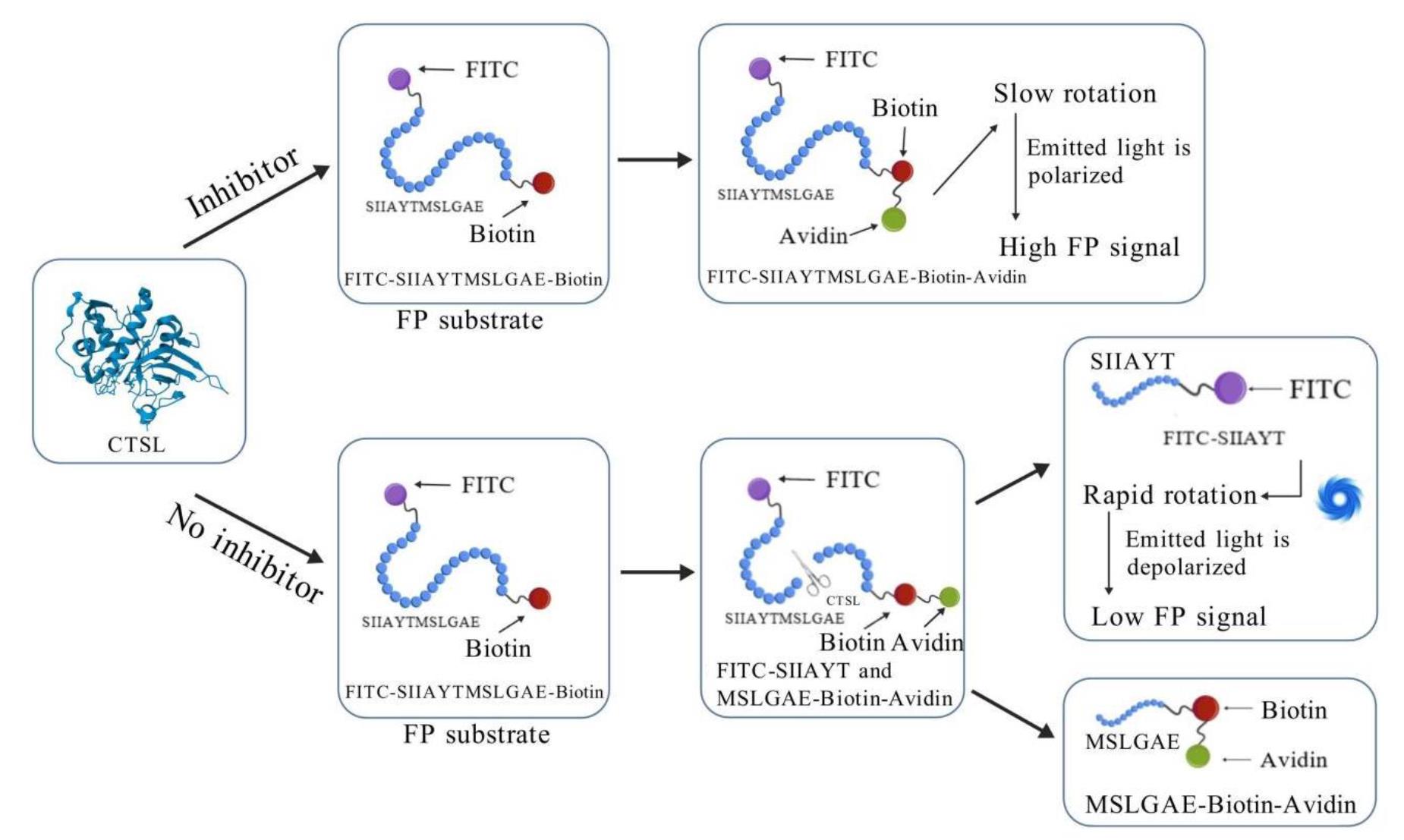
Figure 1. Schematic illustration of fluorescence polarization (FP)-based high-throughput screening (HTS) assay for inhibitors targeting cathepsin L (CTSL). In the FP-based HTS assay, a high-molecular-weight complex is formed through the binding of FITC-SIIAYTMSLGAE-Biotin substrate to avidin, which exhibits slow rotational diffusion, resulting in elevated millipolarization (mP) values. Active CTSL enzymatically cleaves the FP substrate into two fragments: FITC-SIIAYT and MSLGAE-Biotin. The FITC-labeled peptide fragment FITC-SIIAYT, due to its low molecular weight, undergoes rapid rotational motion and displays isotropic emission, thereby generating a reduced FP signal. Consequently, inhibition of CTSL enzymatic activity by bioactive compounds preserves the integrity of the avidin-bound substrate, leading to retention of high mP values. This inverse correlation between protease activity and mP signal provides a robust quantitative readout for identifying and validating CTSL inhibitors in HTS campaigns.
Materials and reagents
Biological materials
1. Escherichia coli (E. coli) DH5α competent cells (TransGen Biotech, catalog number: CD201-01)
2. HEK-293T cells (Sunncell Biotech, catalog number: SNL-015)
Reagents
1. DNA marker (TransGen Biotech, catalog number: BM401-01)
2. Plasmid Extraction kit (TransGen Biotech, catalog number: EM101-02)
3. Protein marker (Meilun Biotech, catalog number: MA0352-1)
4. SARS-CoV-2 S protein (Sino Biological, catalog number: 40591-V08H)
5. CTSL DNA fragment (NCBI reference sequence: NM_001912.4, synthesized by GenScript Biotech)
6. FP substrate: FITC-SIIAYTMSLGAE-Biotin (synthesized by GenScript Biotech)
7. CTSL protein (Sino Biological, catalog number: 10486-H08H)
8. DMEM (Cytiva Company, catalog number: SH30021.01)
9. DMSO (Thermo Fisher Scientific, catalog number: D12345)
10. Kpn I (Biolabs, catalog number: R3142V)
11. Xba I (Biolabs, catalog number: R0145V)
12. FBS (Gibco, catalog number: A5670701)
13. Avidin (Thermo Fisher Scientific, catalog number: 434401)
14. Z-Phe-Tyr-CHO (MedChem Express, catalog number: HY-128140)
15. FreeStyleTM 293 expression medium (Gibco, catalog number: 12338026)
16. Imidazole (VETEC, catalog number: V900153-500G)
17. Yeast extraction (OXODI, catalog number: LP0021T)
18. Agar powder (OXODI, catalog number: 22700041)
19. HisTrapTM chromatography column (Cytiva Company, catalog number: 17524802)
20. Bicinchoninic Acid (BCA) Protein Quantitative kit (Thermo Fisher Scientific, catalog number: 23227)
21. CTSL polyclonal antibody (Thermo Fisher Scientific, catalog number: PA5-119012)
22. Horseradish peroxidase (HRP) labeled goat anti-rabbit IgG (ZSGB Biotech, catalog number: PI-1000)
23. Carbobenzoxy-phenylalanyl-arginyl-7-amino4-methylcoumarin (Z-Phe-Arg-AMC) (Sigma, catalog number: C9521)
24. Kanamycin (Sigma, catalog number: E004000)
25. 7-Amino-4-methylcoumarin (AMC) (Sigma, catalog number: 257370)
26. LipofectamineTM 2000 (Thermo Fisher Scientific, catalog number: 11668019)
27. pCMV3-C-His-NCV (Sino Biological, catalog number: CV015)
28. Tris (Sigma, catalog number: S4762)
29. BSA (Biosharp, catalog number: BL2182A)
30. DTT (Sigma, catalog number: V900830)
31. EDTA tetrasodium salt (Aladdin, catalog number: T161517)
32. 100× penicillin-streptomycin solution (Biosharp, catalog number: BL505A)
33. HRP-conjugated anti-rabbit IgG (GenStar Biosolutions, catalog number: AS00039)
34. Anti-CTSL polyclonal antibody (Solarbio, catalog number: P07711)
35. NaCl (Biosharp, catalog number: BS112-1kg)
36. Tryptone (OXOID, catalog number: LP0042B)
37. Coomassie Brilliant Blue R-250 (Lab Blue, catalog number: G1042)
38. Methanol (Sigma, catalog number: 322415)
39. Glacial acetic acid (Sigma, catalog number:1005706)
40. Supersensitive ECL chemiluminescence solution (Abbkine Scientific, catalog number: BMU102)
41. EDTANa2 (Solarbio, catalog number: E8030-100)
42. Sodium phosphate (J&K Scientific, catalog number: 997519)
43. Opti-MEM (Thermo Fisher Scientific, catalog number: 11058021)
44. Non-fat dry milk (Bio Froxx, catalog number: 1172GR500)
45. Na2HPO4 (SAITONG, catalog number: S10034-500G)
46. KH2PO4 (MACKLIN, catalog number: P815662)
47. KCl (MACKLIN, catalog number: P816348)
48. Tris-HCl (Solarbio, catalog number: T8230)
49. Tween® 20 (MACKLIN, catalog number: T6335-500ml)
50. HEPES (MACKLIN, catalog number: H917470)
51. Brij® 35 (polyepoxyethylene lauryl ether) (Sangon Biotech, catalog number: A610217-0250)
Solutions
1. Phosphate-buffered saline (PBS) (see Recipes)
2. FP assay buffer (see Recipes)
3. Protein loading buffer (see Recipes)
4. Protein elution buffer (see Recipes)
5. Fluorescence-labeled substrate model buffer (see Recipes)
6. LB liquid medium (see Recipes)
7. LB solid medium (see Recipes)
8. Kanamycin stock solution (see Recipes)
9. HEPES (see Recipes)
10. AMC stock solution (see Recipes)
11. Coomassie Brilliant Blue R-250 staining solution (see Recipes)
12. Destaining solution (see Recipes)
13. Tris-Buffered Saline with Tween 20 (TBST) (see Recipes)
Recipes
1. Phosphate-buffered saline (PBS)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 10.14 mM | 1.44 g |
| NaCl | 136.75 mM | 8 g |
| KH2PO4 | 1.76 mM | 0.24 g |
| KCl | 2.68 mM | 0.2 g |
| dH2O | n/a | Up to 1,000 mL |
| Total | n/a | 1,000 mL |
Note: Adjust pH to 7.4 with an electronic pH meter using 1 N HCl or 1 N NaOH. Filter through a 0.22 μm membrane or autoclave (121 °C, 15–20 min) for cell culture use. Store at room temperature (RT) (short-term) or 4 °C (long-term). Check for precipitation before use.
2. FP assay buffer (pH 6.0)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris | 10 mM | 1.21 g |
| NaCl | 50 mM | 2.92 g |
| EDTA tetrasodium salt | 1 mM | 0.45 g |
| DTT | 1 mM | 3.08 g |
| ddH2O | n/a | Up to 1,000 mL |
| Total | n/a | 1,000 mL |
Note: Adjust pH to 6.0 with an electronic pH meter using 1 N HCl or 1 N NaOH.
3. Protein loading buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 500 mM | 14.61 g |
| Imidazole | 20 mM | 0.6808 g |
| PBS | 50 mM | Up to 500 mL |
| Total | n/a | 500 mL |
4. Protein elution buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 500 mM | 14.61 g |
| Imidazole | 400 mM | 13.616 g |
| PBS | 50 mM | Up to 500 mL |
| Total | n/a | 500 mL |
5. Fluorescence-labeled substrate model buffer (pH 5.5)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DTT | 6 mM | 9.24 g |
| Brij® 35 (polyepoxyethylene lauryl ether) | 0.05% | 0.5 g |
| EDTANa2 | 0.05% | 0.5 g |
| Sodium phosphate solution | 100 mM | Up to 1,000 mL |
| Total | n/a | 1,000 mL |
6. LB liquid medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 1.0% | 5 g |
| Tryptone | 1.0% | 5 g |
| Yeast extraction | 0.5% | 2.5 g |
| Deionized water | n/a | Up to 500 mL |
| Total | n/a | 500 mL |
Note: Autoclave at 121 °C for 15 min.
7. LB solid medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 1.0% | 5 g |
| Tryptone | 1.0% | 5 g |
| Yeast extraction | 0.5% | 2.5 g |
| Agar | 1.5% | 7.5 g |
| Deionized water | n/a | Up to 500 mL |
| Total | n/a | 500 mL |
Note: Autoclave at 121 °C for 15 min. Cool medium to ~55 °C. After adding kanamycin with a final concentration of 100 μg/mL, pour 15–20 mL/plate into sterile Petri dishes under aseptic conditions. Let plates solidify at RT for 30 min. Store inverted at 4 °C for about 1 week.
8. Kanamycin stock solution (100 mg/mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Kanamycin powder | 100 mg/mL | 100 mg |
| Deionized water | n/a | Up to 1 mL |
| Total | n/a | 1 mL |
Note: Sterilize by filtration through a 0.22 μm membrane filter and store at -20 °C for future use.
9. HEPES (pH 7.0)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| HEPES | 10 mM | 2.38 g |
| NaCl | 50 mM | 2.92 g |
| EDTA | 1 mM | 0.29 g |
| DTT | 1 mM | 0.15 g |
| Total | n/a | 1 mL |
10. AMC stock solution (10 mM)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| AMC | 10 mM | 1.75 g |
| DMSO | n/a | 1 mL |
| Total | n/a | 1 mL |
Note: Aliquot and store at -20 °C, protecting from light. Dilute with HEPES to the required concentration before use.
11. Coomassie Brilliant Blue R-250 staining solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Coomassie Brilliant Blue R-250 | 0.1% w/v | 0.25 g |
| Methanol (or ethanol) | n/a | 90 mL |
| dH2O | n/a | 90 mL |
| Glacial acetic acid | n/a | 20 mL |
| Total | n/a | 200 mL |
Note: Dissolve 0.25 g (0.1% w/v) Coomassie Brilliant Blue R-250 in a mixture of 90 mL methanol (or ethanol) and 90 mL dH2O. Then, add 20 mL of glacial acetic acid.
12. Destaining solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Methanol (or ethanol) | 40% | 400 mL |
| Glacial acetic acid | 10% | 100 mL |
| dH2O | 50% | 500 mL |
| Total | n/a | 1,000 mL |
13. TBST (pH 7.4)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris-HCl | 20 mM | 3.15 g |
| NaCl | 150 mM | 8.77 g |
| Tween® 20 | 0.1% | 1 mL |
| Deionized water | n/a | Up to 1,000 mL |
| Total | n/a | 1,000 mL |
Note: Adjust pH to 6.0 with an electronic pH meter using 1 N HCl or 1 N NaOH. Stable at RT for weeks (if sterile). For long-term use, store at 4 °C.
Laboratory supplies
1. Black walled half-bottom plate (Corning, catalog number: 3694)
2. 1.5 mL microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 05-408-129)
3. Pipette tips (1,000, 200, and 10 μL) (Thermo Fisher Scientific, catalog numbers: 02-707-401, 02-708-416, 02-707-437)
4. 200 mL shaking flask for Expi293F (Thermo Fisher Scientific, catalog number: PBV12-5)
5. Micropipettes (Thermo Fisher Scientific, catalog number: F167380)
6. 10 cm culture dish (LABSELECT, catalog number: 12311)
7. 0.22 μm membrane filter (Biosharp, catalog number: BS-PES-22)
8. Cell scraper (LABSELECT, catalog number: 1900)
9. Ultrafiltration tube,10 kDa MWCO (Millipore, catalog number: UFC9010)
Equipment
1. Centrifuge (Hettich, catalog number: Mikro 12-24)
2. Ultrasonic crusher (Scientz Biotechnology, catalog number: SCIENTZ-II D)
3. Water bath (Julabo, catalog number: TW12)
4. Visible UV detector (Shimadzu, catalog number: SPD-20A)
5. In-line degasser (Shimadzu, catalog number: DGU-20A)
6. Surface plasmon resonator (Reichert, catalog number: 2SPR)
7. Ultra-low temperature freezer (SANYO, catalog number: MDF-U3286S)
8. Ultra-clean workbench (JiangSu SuJing, catalog number: BCM-1000A)
9. Water purifier (Millipore, catalog number: Milli-Q)
10. High-content imaging systems (PerkinElmer, catalog number: Operetta CLS)
11. Protein electrophoresis tank (Bio-Rad, catalog number: 1658002)
12. Microscope (Olympus, catalog number: CKX41SF)
13. Incubator (Shanghai Jiecheng, catalog number: HI-80V)
14. Electronic balance (Mettler Toledo, catalog number: AL104)
15. Electronic pH meter (Sartorius, catalog number: PB-10)
16. Microplate reader (PerkinElmer, catalog number: Enspire 2104 Multilabel Reader)
17. Tabletop centrifuge (Eppendorf, catalog number: 5405000441)
18.ChemiDocTM MP imaging system (Bio-Rad, catalog number: 12003154)
19. Variable mode imager (Tanon, catalog number: Tanon 5200)
20. PH meter (Shanghai Yidian Scientific Instrument Co., Ltd, catalog number: PHS-2F)
21. ÄKTA chromatography system (GE, catalog number: AKTA Explorer 100)
22. Microscope (Olympus, catalog number: DP74)
23. Variable mode imager (Tanon, catalog number: 5200)
Software and datasets
1. GraphPad Prism 9.0 (GraphPad Software, San Diego, USA)
2. CellSens Standard (Olympus, Tokyo, Japan)
Procedure
文章信息
稿件历史记录
提交日期: Apr 10, 2025
接收日期: Jun 24, 2025
在线发布日期: Jul 11, 2025
出版日期: Jul 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Guo, K., You, B., Zhou, W., Li, Y., Wang, Z., Zhang, J. and Si, S. (2025). Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L. Bio-protocol 15(14): e5400. DOI: 10.21769/BioProtoc.5400.
分类
分子生物学 > 蛋白质 > 表达
生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link