- EN - English
- CN - 中文
Fixation and Expansion Microscopy of Xenopus Egg Extract Spindles
非洲爪蟾卵提取液中纺锤体的固定与膨胀显微成像方法
发布: 2025年07月20日第15卷第14期 DOI: 10.21769/BioProtoc.5396 浏览次数: 1185
评审: Komuraiah MyakalaAnonymous reviewer(s)
Abstract
In vitro systems based on Xenopus egg extracts have elucidated many aspects of spindle assembly. Still, numerous unknowns remain, particularly concerning the variation in spindle morphologies. The X. laevis and X. tropicalis egg extract systems, which recapitulate diverse spindle sizes and architectures, serve as ideal tools to investigate the regulation of spindle morphometrics. However, fully understanding spindle architectural differences is hindered by the spindle's size and high microtubule density. Indeed, classical fluorescence microscopy lacks the resolution to detail the organization of spindle microtubules, and although electron tomography can distinguish individual microtubules, segmenting thousands of microtubules and tracking them across dozens of sections remains an unachieved challenge. Therefore, we set out to apply expansion microscopy to the study of Xenopus egg extract spindles. During this process, we realized that optimizing spindle fixation as well was crucial to preserve microtubule integrity. Here, we present an optimized fixation and expansion microscopy protocol that enables the study of spindle architecture in egg extracts of both X. laevis and X. tropicalis. Our method retains the fluorescence of rhodamine tubulins added to the extracts and allows for both pre- and post-expansion immunofluorescence analysis.
Key features
• Expansion of optimally fixed spindle assembled from egg extracts of X. leavis, X. tropicalis, and possibly others.
• Retains the fluorescence of the rhodamine-tubulin that copolymerizes with endogenous Xenopus tubulin within spindle microtubules, allowing their imaging without immunolabeling.
• Compatible with both pre- and post-expansion immunolabeling to increase labeling possibilities.
• Optimized spindle fixation that best preserves microtubule integrity for expansion and can also be used without expansion for regular immunofluorescence experiments.
Keywords: Xenopus (非洲爪蟾)Graphical overview
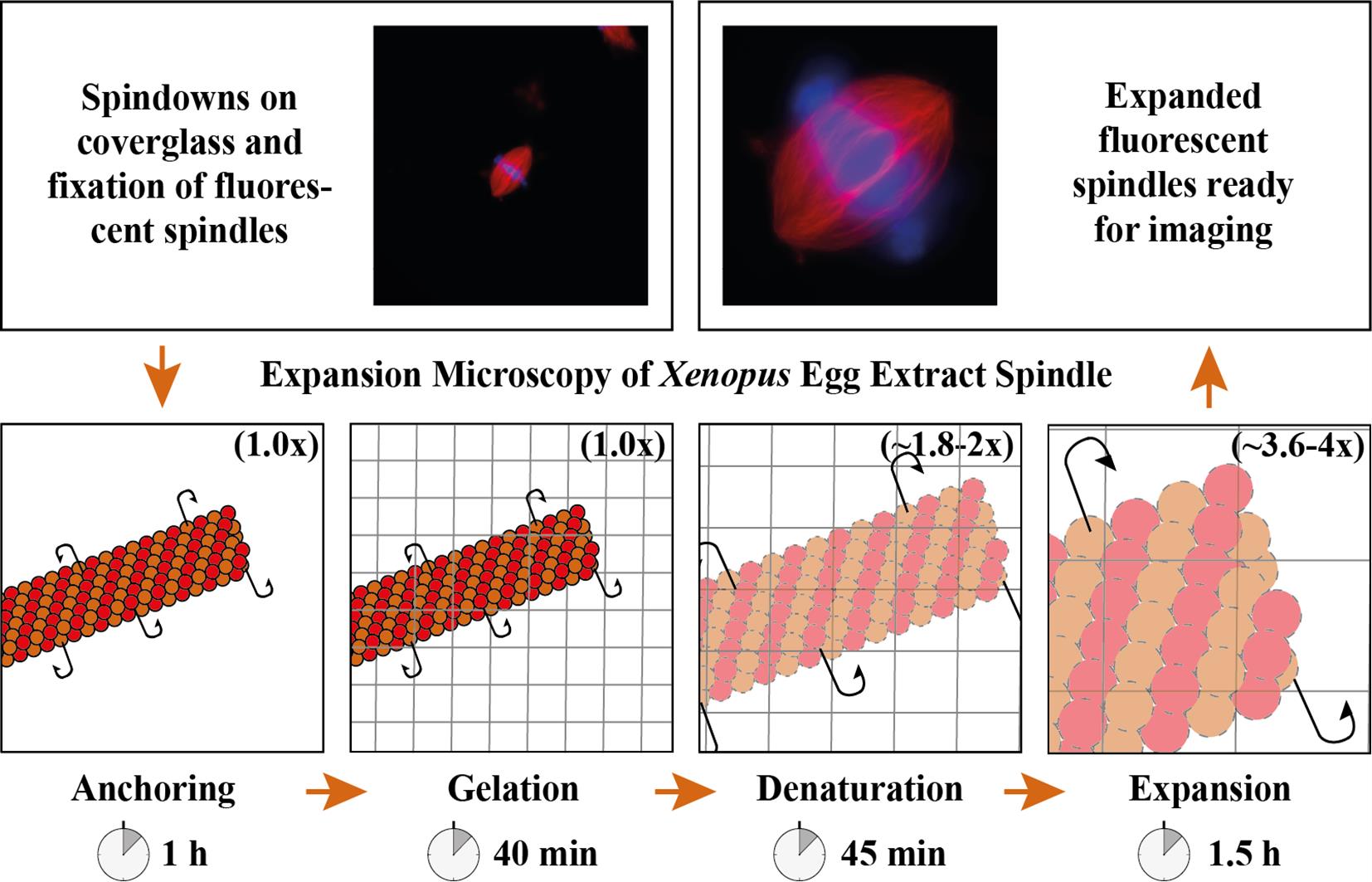
Expansion microscopy of Xenopus egg extract spindles. Spindle assembled in the presence of rhodamine-labeled tubulin can be expanded up to 4 times following anchoring, gelation, denaturation, and expansion to be imaged at higher resolution.
Background
Mitosis is crucial to the cell cycle, ensuring the accurate segregation of replicated chromosomes to daughter cells. This process relies on the spindle, a dynamic, microtubule-based, bipolar structure, which aligns chromosomes at the metaphase plate and separates sister chromatids during anaphase. Studying spindle assembly and function is particularly relevant because chromosome segregation defects underlie various human diseases [1]. Spindle size, architecture, and dynamics vary significantly among different cell types and organisms [2], optimizing its function. However, the establishment of specific spindle morphologies remains poorly understood despite their importance for proper spindle function.
In vitro systems using Xenopus egg extracts have elucidated many aspects of mitosis and spindle assembly [3], but many unknowns remain, especially regarding spindle morphology variation. The X. laevis and X. tropicalis egg extract systems, which recapitulate different spindle sizes and architectures, are ideal tools to explore the basis of vertebrate spindle architecture and size regulation [4]. Previous studies have shown that X. tropicalis spindles are not only shorter than X. laevis spindles [5] but also exhibit distinct microtubule bundling and density patterns [6] as well as more robust kinetochore fibers [7]. However, the high microtubule density in these spindles makes it impossible to resolve individual filaments using classical fluorescence microscopy. Super-resolution techniques have significantly improved microscopy resolution, even reaching the nanometer scale [8,9]. However, these methods often require specific fluorophores, sample environments, and, most importantly, equipment. Electron tomography provides the best resolution, allowing imaging and tracking of individual microtubules. It has been used to reconstruct spindles from various species, including Saccharomyces cerevisiae and Ashbya gossypii [10], Caenorhabditis elegans [11], and HeLa cells [12]. While this technique is labor-intensive and time-consuming, the largest reconstructed spindles are not even half the size of the X. laevis spindle, which still keeps it out of reach.
To gain greater architectural information, we developed an expansion microscopy (ExM) protocol optimized for Xenopus egg extract spindles [13]. Combining aspects of original ExM protocols [14,15], the MAP protocol [16], and the widely-used U-ExM protocol [17], our method maintains the fluorescence of the rhodamine-tubulin, which copolymerizes with endogenous Xenopus tubulin, allowing microtubule imaging without immunolabeling. Since not all antibodies are suitable for post-expansion immunolabeling, this protocol retains the possibility of pre-expansion immunolabeling, addressing the difficulty of finding effective antibodies for Xenopus. By enhancing resolution and minimizing artifacts, our ExM approach, with extended immunolabeling capacities, provides a new and powerful means to explore microtubule and assembly factor organization to better understand spindle architecture and morphology. Although our protocol is now optimized for the Xenopus egg extract systems, it can likely be adapted for structures assembled in extracts beyond spindles and for various other frog species [4,18].
Materials and reagents
Biological materials
1. Spindles assembled from Xenopus egg extracts, prepared as previously described [19,20]
Reagents
1. 1,4-Piperazinediethanesulfonic acid (PIPES) (Sigma-Aldrich, Merck, catalog number: P6757-500G)
2. Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) (Sigma-Aldrich, Merck, catalog number: E3889)
3. Magnesium chloride 2 M solution (MgCl2) (Sigma-Aldrich, Merck, catalog number: 68475-100ML-F)
4. Potassium hydroxide (KOH) (Sigma-Aldrich, Merck, catalog number: P5958-500G)
5. Tris base (Euromedex, catalog number: 200923-A)
6. Glycerol (Sigma-Aldrich, Merck, catalog number: G5516-1L)
7. Methanol (Sigma-Aldrich, Merck, catalog number: 322415-250ML) stored at -20 °C
8. Triton X-100 (Sigma-Aldrich, Merck, catalog number: T8787)
9. Vaseline (Sigma-Aldrich, Merck, catalog number: 16415)
10. Lanolin (Sigma-Aldrich, Merck, catalog number: L7387)
11. Paraffin wax (Sigma-Aldrich, Merck, catalog number: 76242)
12. 6-((acryloyl)amino)hexanoic acid, succinimidyl ester (AcX) (Thermo Fisher Scientific, catalog number: A20770)
13. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650-5X5ML)
14. Sodium acrylate (Sigma-Aldrich, Merck, catalog number: 408220-100G)
15. Acrylamide 40% solution (Interchim, catalog number: UP873376 ACRYL-40)
16. N,N’-methylenebisacrylamide 2% solution (Sigma-Aldrich, Merck, catalog number: M1533)
17. N,N,N′,N′-Tetramethylethylenediamine (TEMED) (Sigma-Aldrich, Merck, catalog number: T9281-25ML)
18. Ammonium persulfate (APS) (Sigma-Aldrich, Merck, catalog number: A3678-25G)
19. 10× Phosphate buffered saline (PBS) (Euromedex, catalog number: ET330)
20. Sodium dodecyl sulfate 20% solution (SDS) (Sigma-Aldrich, Merck, catalog number: 05030-500ML-F)
21. Sodium chloride (NaCl) (Sigma-Aldrich, Merck, catalog number: 31434-1KG-M)
22. Bovine serum albumin (BSA) (Euromedex, catalog number: 04-100-812-C)
23. IGEPAL® CA-630 (Sigma-Aldrich, Merck, catalog number: I8896-50ML)
24. Hoechst 33342 (Sigma-Aldrich, Merck, catalog number: B2261-25MG)
Solutions
1. 1 M K-PIPES (see Recipes)
2. 0.1 M K-EGTA (see Recipes)
3. 5× BRB80 (see Recipes)
4. Spindle dilution buffer (see Recipes)
5. Spindle cushion buffer (see Recipes)
6. PBS (see Recipes)
7. PBSI (see Recipes)
8. AcX stock solution (see Recipes)
9. Anchoring solution (see Recipes)
10. 1 M Tris base (see Recipes)
11. 4 M NaCl solution (see Recipes)
12. Denaturation buffer (see Recipes)
13. PBT (see Recipes)
14. Sodium acrylate 38% solution (see Recipes)
15. Monomer solution (see Recipes)
16. 10× APS stock solution (see Recipes)
17. Hoechst stock solution (see Recipes)
18. Valap sealant (see Recipes)
Recipes
1. 1 M K-PIPES
| Reagent | Final concentration | Amount |
|---|---|---|
| PIPES | 1 M | 302.37 g |
| KOH | n/a | to pH 6.8 |
| Water (MilliQ) | n/a | to 1 L |
| Total | n/a | 1 L |
To prepare this buffer, begin by adding the powder to 700–800 mL of water in a beaker. Stir the solution continuously while gradually increasing the pH by carefully adding KOH pellets. As the pH rises, the powder will begin to dissolve. Once most of the powder has dissolved, continue adding the pellets one at a time. Wait until the powder is fully solubilized before measuring the pH. At this stage, use a pH meter equipped with temperature compensation. Adjust the pH to 7.8 by first using KOH pellets one by one up to pH 7, then fine-tune the final value with a 10 N KOH solution. Once the target pH is reached, transfer the solution to a measuring cylinder and bring the final volume up to 1 L with water. Filter-sterilize and store at 4 °C.
2. 0.1 M K-EGTA
| Reagent | Final concentration | Amount |
|---|---|---|
| EGTA | 0.1 M | 19.02 g |
| KOH | n/a | to pH 7.8 |
| Water (MilliQ) | n/a | to 500 mL |
| Total | n/a | 500 mL |
To prepare this buffer, begin by adding the powder to 350–400 mL of water in a beaker. Stir the solution continuously while gradually increasing the pH by carefully adding KOH pellets. As the pH rises, the powder will begin to dissolve. Once most of the powder has dissolved, continue adding the pellets one at a time. Wait until the powder is fully solubilized before measuring the pH. At this stage, use a pH meter equipped with temperature compensation. Adjust the pH to 7.8 by first using KOH pellets one by one up to pH 7, then fine-tune the final value with a 10 N KOH solution. Once the target pH is reached, transfer the solution to a measuring cylinder and bring the final volume up to 500 mL with water. Filter-sterilize and store at 4 °C.
3. 5× BRB80
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M K-PIPES | 400 mM | 400 mL |
| 0.1 M K-EGTA | 5 mM | 50 mL |
| 2 M MgCl2 | 5 mM | 2.5 mL |
| KOH | n/a | to pH 6.8 |
| Water | n/a | To 1 L |
| Total | n/a | 1 L |
Filter-sterilize and store at 4 °C.
4. Spindle dilution buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Glycerol | 30% | 30 mL |
| 5× BRB80 | 1× | 20 mL |
| Triton X-100 | 0.5% | 500 μL |
| Water (MilliQ) | n/a | to 100 mL |
| Total | n/a | 100 mL |
Filter-sterilize and store at 4 °C.
5. Spindle cushion buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Glycerol | 40% | 200 mL |
| 5× BRB80 | 1× | 100 mL |
| Water (MilliQ) | n/a | to 500 mL |
| Total | n/a | 500 mL |
Filter-sterilize and store at 4 °C.
6. PBS
| Reagent | Final concentration | Amount |
|---|---|---|
| 10× PBS | 1× | 100 mL |
| Water (MilliQ) | n/a | 900 mL |
| Total | n/a | 1 L |
Filter-sterilize and store at room temperature.
7. PBSI
| Reagent | Final concentration | Amount |
|---|---|---|
| IGEPAL® CA-630 | 0.1% | 10 μL |
| PBS | n/a | 9.99 mL |
| Total | n/a | 10 mL |
Prepare fresh at room temperature. Place on a rotator and allow time for the IGEPAL to get into solution.
8. AcX stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| AcX | 10 mg/mL | 5 mg |
| DMSO | n/a | 500 μL |
| Total | n/a | 500 μL |
Prepare 10 μL aliquots and store at -20 °C.
9. Anchoring solution
| Reagent | Final concentration | Amount |
|---|---|---|
| AcX stock solution | 0.1 mg/mL | 10 μL |
| PBS | n/a | 990 μL |
| Total | n/a | 1 mL |
Prepare fresh. Freeze the rest at -20 °C and thaw it for one more use.
10. 1 M Tris base
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris base | 1 M | 121.1 g |
| HCl | n/a | to pH 8 |
| Water (MilliQ) | n/a | to 1 L |
| Total | n/a | 1 L |
Filter-sterilize and store at room temperature.
11. 4 M NaCl solution
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 4 M | 58.44 g |
| Water (MilliQ) | n/a | to 250 mL |
| Total | n/a | 250 mL |
Filter-sterilize and store at room temperature.
12. Denaturation buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M Tris base | 0.1% | 2 mL |
| SDS 20% solution | 200 mM | 11.52 mL |
| 4 M NaCl | 200 mM | 2 mL |
| Water (MilliQ) | n/a | 24.48 mL |
| Total | n/a | 40 mL |
Prepare fresh at room temperature. Place on a rotator and allow time for the solution to homogenize. Count 40 mL per gel.
Note: The SDS precipitates at temperatures below 15 °C and at even higher temperatures when combined with high salt concentration, such as in the denaturation buffer. Therefore, make sure that the room temperature is not too low to prevent the precipitation of the SDS.
13. PBT
| Reagent | Final concentration | Amount |
|---|---|---|
| Triton X-100 | 0.1% | 100 μL |
| PBS | n/a | 99.9 mL |
| Total | n/a | 100 mL |
Prepare fresh at room temperature. Place on a rotator and allow time for the Triton X-100 to get into solution. Count ~100 mL per gel.
14. Sodium acrylate 38% solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium acrylate | 38% (wt/wt) | 19 g |
| Water (MilliQ) | n/a | 31 mL |
| Total | n/a | 31 mL |
Prepare 1.5 mL aliquots and store at -20 °C. Never refreeze; always take a new aliquot.
Note: Sodium acrylate sometimes comes with a variable purity level, which can affect performance. When preparing the sodium acrylate stock solution, check that it is colorless under normal room lighting. The solution should be as clear as possible. If the solution has a yellow tint, discard the solution and order a new batch of sodium acrylate.
15. Monomer solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 10× PBS | 1× | 200 μL |
| Sodium acrylate 38% solution | 19% | 1 mL |
| Acrylamide 40% solution | 10% | 500 μL |
| N,N’-methylenebisacrylamide 2% solution | 0.1% | 100 μL |
| Water (MilliQ) | n/a | 200 μL |
| Total | n/a | 2 mL |
Prepare 85 μL aliquots and store at -20 °C. Never refreeze; always take a new aliquot. Count one per gel.
16. 10× APS stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| APS | 10% | 1 g |
| Water (MilliQ) | n/a | to 10 mL |
| Total | n/a | 10 mL |
Prepare 50 μL aliquots and store at -20 °C. Never refreeze; always take a new aliquot.
17. Hoechst stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Hoechst 33342 | 10 mg/mL | 25 mg |
| Water (MilliQ) | n/a | 2.5 mL |
| Total | n/a | 2.5 mL |
Prepare 200 μL aliquots and store at -20 °C for long-term storage. When thawing an aliquot, keep it at 4 °C until finished.
18. Valap sealant
| Reagent | Final concentration | Amount |
|---|---|---|
| Vaseline | 1/3 | 10 g |
| Lanolin | 1/3 | 10 g |
| Paraffin wax | 1/3 | 10 g |
| Total | n/a | 30 g |
Heat the mixture in a 100 mL glass beaker, just warm enough so that it liquefies. Stir occasionally. Do not overheat. Its color should stay golden yellow. Poor into a 50 mL screw-capped tube and keep at room temperature.
Laboratory supplies
1. 1.5 mL Eppendorf tubes (Eppendorf, catalog number: 0030123611)
2. Rapid-flow filters (Nalgene, Thermo Fischer Scientific, catalog number: 597-4520)
3. Pipette tips (TipOne)
4. 15 mL round-bottom glass centrifuge tube, high-strength (Kimble, catalog number: 45500-15)
5. Teflon adaptor for coverslips (homemade, see technical drawing in Figure 1A)
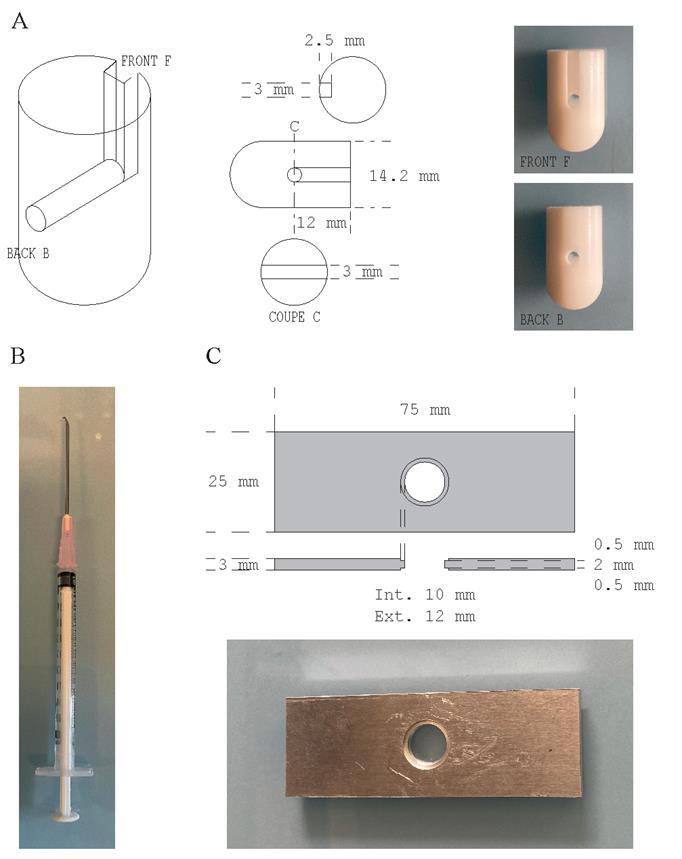
Figure 1. Homemade laboratory supplies. (A) Coverslip adaptor engineered from a Teflon rod according to the technical drawing presented (modified from Gogendeau et al. [21]). (B) Metal hook made from an 18 G needle tip bent as a hook and attached to a 1 mL syringe. (C) Slide to image coverslips engineered from a 3-mm-thick aluminum sheet according to the technical drawing presented.
6. Round 12 mm glass coverslips (Menzel-Gläser, catalog number: CB00120RA020)
7. Adapter rubber sleeve (Kimble, catalog number: 45550-15)
8. 1 mL syringe (Terumo, catalog number: MDSS01SE)
9. 18 G needle (Terumo, catalog number: AN*1838R1)
10. Metal hook (made from an 18G needle tip bent as a hook and attached to a 1 mL syringe; see Figure 1B)
11. Forceps (Dumont, catalog number: #55)
12. Parafilm (Bemis, catalog number: PM996)
13. Kimwipes (Kimtech, Kimberly-Clark, catalog number: 05511)
14. Metal slide for imaging spindles before expansion (homemade, see technical drawing in Figure 1C)
15. Test tube plastic rack (Nalgene, Thermo Fischer Scientific, catalog number: 5970-0530)
16. 6-well plates (Falcon, catalog number: 353046)
17. 100 mL glass beaker
18. 5 cm glass Petri dish
19. 14 cm plastic Petri dish
20. Microscope glass slides 76 mm × 26 mm (Menzel-Gläser)
21. #1.5, 24 mm × 60 mm microscope glass coverslips (Deckgläser)
22. Glass bottom dishes, Poly-d-lysine coated (MatTek, catalog number: P35GC-1.0-14C)
Equipment
1. High-speed centrifuge (Beckman Coulter, model: Avanti JXN-26)
2. Swinging bucket rotor (Beckman Coulter, model: JS13-1)
3. Incubator (Binder, model: B240), constantly set to 37 °C
4. Water bath (Grant, model: GD100-S18), to be set to 95 °C
5. Rotator (Grant, model: PS-3D)
Procedure
文章信息
稿件历史记录
提交日期: Apr 22, 2025
接收日期: Jun 22, 2025
在线发布日期: Jul 10, 2025
出版日期: Jul 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Guilloux, G., Kitaoka, M., Mocaer, K., Heichette, C., Duchesne, L., Heald, R., Pecot, T. and Gibeaux, R. (2025). Fixation and Expansion Microscopy of Xenopus Egg Extract Spindles. Bio-protocol 15(14): e5396. DOI: 10.21769/BioProtoc.5396.
- Guilloux, G., Kitaoka, M., Mocaer, K., Heichette, C., Duchesne, L., Heald, R., Pecot, T. and Gibeaux, R. (2025). Optimized expansion microscopy reveals species-specific spindle microtubule organization in Xenopus egg extracts. Mol Biol Cell. 36(6): ar73. https://doi.org/10.1091/mbc.E24-09-0421
分类
细胞生物学 > 细胞结构 > 微管
细胞生物学 > 细胞成像 > 超分辨率成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link