- EN - English
- CN - 中文
Using Beads as a Focus Fiduciary to Aid Software-Based Autofocus Accuracy in Microscopy
利用微珠作为聚焦参照提高显微成像自动对焦精度
发布: 2025年07月20日第15卷第14期 DOI: 10.21769/BioProtoc.5388 浏览次数: 1100
评审: Anonymous reviewer(s)
Abstract
Brightfield microscopy is an ideal application for studying live cell systems in a minimally invasive manner. This is advantageous in long-term experiments to study dynamic cellular processes such as stress response. Depending on the sample type and preparation, the inherent qualities of brightfield microscopy, being very low contrast, can contribute to technical issues such as focal drift, sequencing lags, and complete failure of software autofocus systems. Here, we describe the use of microbeads as a focus aid for long-term live cell imaging to address these autofocus issues. This protocol is inexpensive to implement, without extensive additional sample preparation, and can be used to capture focused images of transparent cells in a label-free manner. To validate this protocol, a widefield inverted microscope was used with software-based autofocus to image overnight in time-lapse format, demonstrating the use of the beads to prevent focal drift in long-term experiments. This improves autofocus accuracy on relatively inexpensive microscopes without using hardware-based focus aids. To validate this protocol, the KNIME logistics software was used to train a random forest model to perform binary image classification.
Key features
• Label-free live cell imaging in time-lapse format.
• Troubleshooting software autofocus for brightfield mode.
Keywords: Live-cell imaging (活细胞成像)Graphical overview
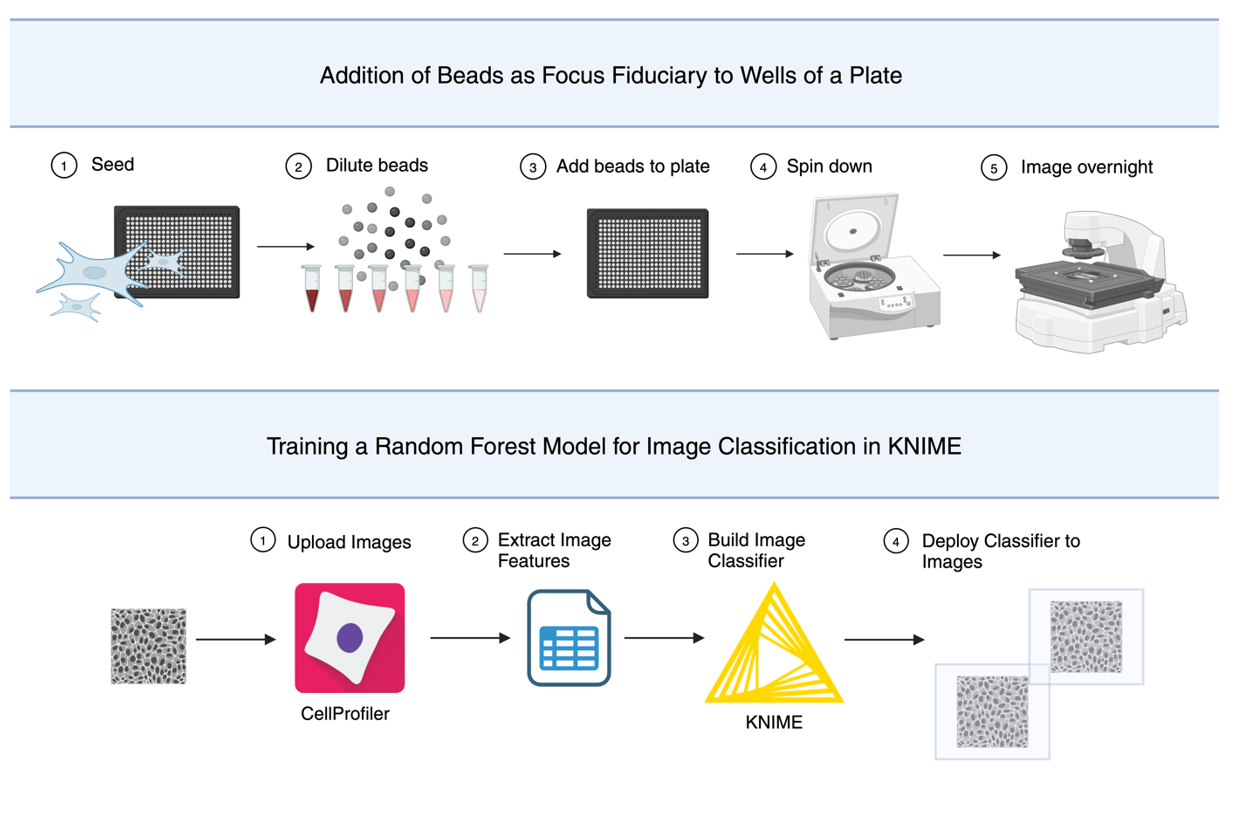
Background
In fluorescence microscopy, fluorescent stains and probes can be used to highlight subcellular structures. The caveats of using fluorescent microscopy, such as photobleaching and phototoxicity, are well described [1]. Phototoxicity refers to the process where typical wavelengths that are used in fluorescence imaging generate excessive reactive oxygen species (ROS), which can lead to DNA damage [2]. This phenomenon is dependent on wavelength and length of exposure to cells [2]. Intracellular ROS production can be buffered by cell antioxidant molecules and enzymes, although in fluorescent imaging, ROS buffering capacities can be exceeded [3,4]. Photobleaching refers to the destruction of a fluorophore, such as a fluorescent protein (i.e., GFP), in the excited state that leads to depletion of signal over time; this can result in the production of ROS and subsequent photobleaching [1,5].
Commonly used stains such as DRAQ5 and Hoechst that highlight chromatin have demonstrated cytotoxic effects in live-cell imaging [6,7]. DRAQ5 dye alters the dynamics and localization of critical proteins involved in DNA transcription, replication, and repair; Hoechst can cause cell cycle arrest or delay of the G2 phase [6,7]. Consideration must be taken when designing experiments to minimize the confounding effects of potentially cytotoxic stains (phototoxicity and photobleaching), especially in long-term acquisitions. This is necessary as artifacts may appear from sample preparation and/or in imaging, and this becomes increasingly important when evaluating the effects of cell treatments.
Time-lapse experiments are easily susceptible to phototoxicity. Cell migration is reduced at high fluorescent light doses compared with cells imaged in brightfield [8]. Time resolution and/or light dose must be compromised to reduce phototoxicity using fluorescent microscopy, making this unsuitable to follow rapid cellular activities [9]. In contrast, brightfield microscopy is label-free, making this technique less invasive and relatively inexpensive. This is an ideal application for studying live-cell systems and processes that are sensitive to oxidative stress caused by elevated ROS, such as mitosis [4,10]. Additionally, information that may differ from fluorescent images, such as cell texture, can be acquired simultaneously with structural information to create novel morphological profiles [11].
Autofocus combines search algorithms and focus metrics to identify the sharpest image across the entire focal depth [12,13]. In automated microscopy, focus metrics are contrast-based algorithms applied to each image when scanning the focal depth. Plotting the magnitude of the focus function creates a focal curve where the most focused image is the global maximum [12]. Ideally, the range of the focus curve is narrow, creating a single defined global maximum with few, if any, local maxima [10]. In practice, when evaluated across different sample types, commonly used focus metrics are far from robust, and the focal curve is not unimodal [14–16].
Confounding the susceptibility of focus metrics to failure, search algorithms are another possible source of error in autofocus. These algorithms represent optimization problems, where the most focused image must be defined accurately and rapidly. Global search is a commonly used search algorithm that samples the entire focal depth but is computationally slow. To optimize speed, the hill climbing method is a type of binary search using a combination of fine and rough focusing; however, it can incorrectly select a local maximum as the most focused image [16]. The susceptibility to failure of autofocus systems appears frequently in the presence of plate scratches, debris, noise, lack of high-frequency content (i.e., few cells, transparent cells), or uneven illumination [14,15]. High-frequency content is associated with sharp objects. Contrast-based autofocus systems identify the global maximum by comparing sharpness across the focal depth [14].
Autofocus algorithms have been evaluated and implemented effectively with fluorescence microscopy, due to the inherent high signal-to-noise ratio [17]. Despite mitigating many drawbacks associated with fluorescence microscopy, the qualities of brightfield microscopy make it more susceptible to autofocus problems. For example, imaging transparent cells such as fibroblasts produces images with low signal-to-noise ratios and makes identifying the correct focal plane by autofocus more error-prone.
Our goal was to utilize an inverted widefield microscope with software autofocus to capture mitotic events in hTERT immortalized patient-derived fibroblasts, also known as TruHD cells [18], to study the impact of Huntington’s disease-associated mutations on cell growth. Since increased cell stress and sensitivity to DNA damage have been demonstrated in TruHD cells [18], we wished to mitigate additional cell stress by choosing to collect brightfield stain-free images. However, we found the software-based autofocus insufficient for use in the transmitted light channel specifically (Figure 1). This failure could not be resolved by imaging in phase contrast or by selecting an alternative built-in autofocus algorithm. In collecting temporal data over time without the aid of autofocus, we observed drift from the manually set focus over time, even while using stains, demonstrating that a working autofocus is necessary to acquire quality movies. In this paper, we describe in detail the use of microbeads as an aid for focusing on the transmitted light channel to acquire images of live cells in time-lapse.
This method is not only effective in eliminating long-term focus drift; in multi-well plates, placing beads in wells adjacent to wells without beads aids focus accuracy. In place of tedious manual annotation of over 3,700 images collected using this protocol, a random forest model was trained. The model was used to perform unbiased binary classification, identifying images as focused or unfocused. The classifier is not required to use beads as a focal fiduciary; however, this was essential to validate this method. This protocol will briefly describe training the machine learning algorithm using the data analysis platform, KoNstanz Information MinEr, or KNIME ( https://www.knime.com/).
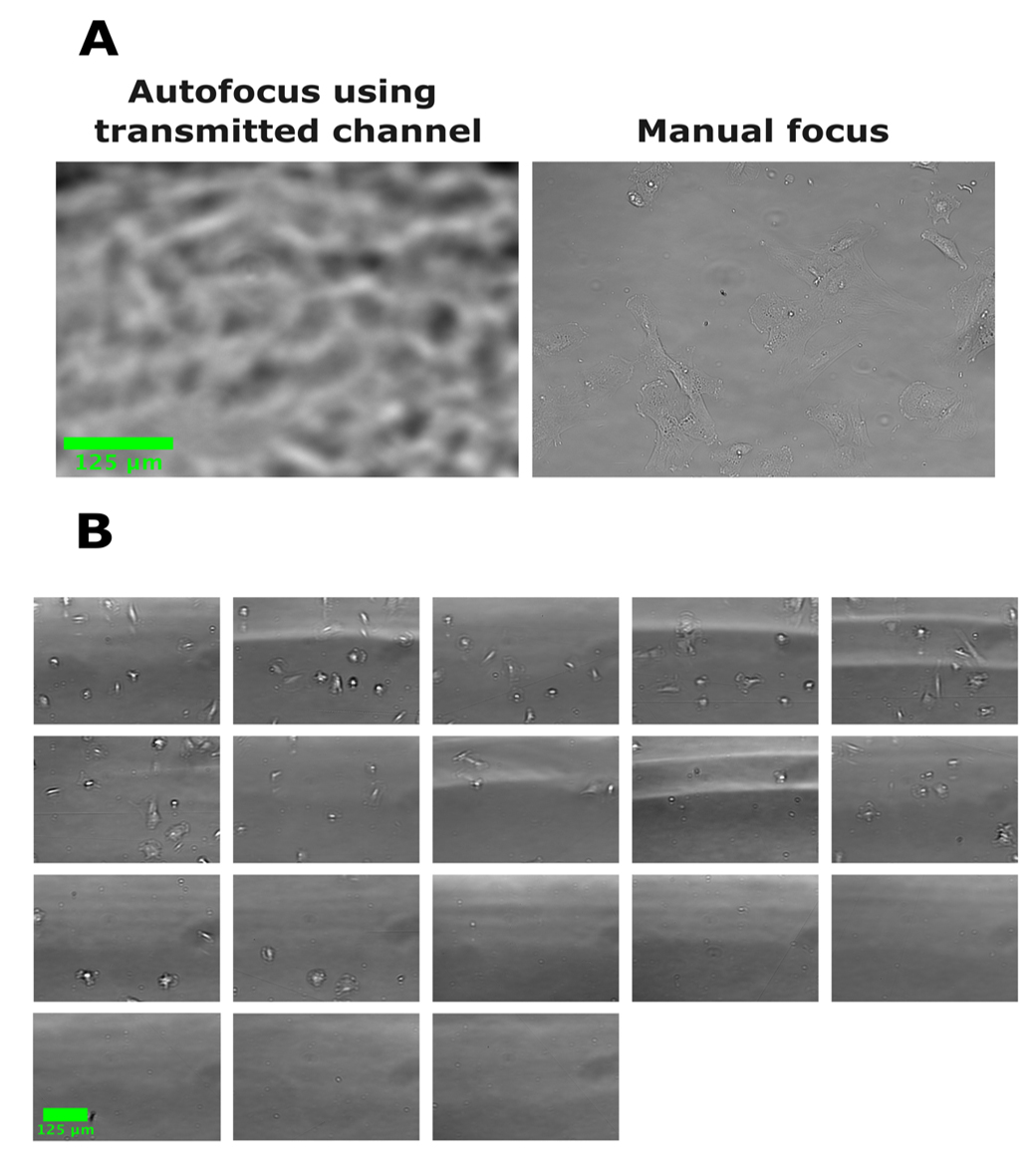
Figure 1. Software autofocus can fail when acquiring brightfield images. In all images, human fibroblasts were seeded in a 384-well plate and images were acquired with a 20× objective Plan fluorite (Evos, NA = 0.5). (A) Example brightfield image acquired using autofocus compared with an image focused manually. Autofocus fails to find the correct plane of focus based on the transmitted light channel. (B) Images acquired of other wells of the same plate from (A) in a single automated scan using autofocus. All images show the failure of autofocus using the transmitted channel to focus.
Materials and reagents
Biological materials
1. TruHD Cells [16]
Reagents
1. Minimal essential media (MEM), 1× (Gibco, catalog number: 10370-021)
2. Fetal bovine serum (Wisent, catalog number: 098450)
3. Deionized microfiltered water
Solutions
1. Supplemented media (see recipes)
Recipes
1. Supplemented media
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MEM 1× | n/a | 500 mL |
| Fetal bovine serum | 13% | 75 mL |
| Total | n/a | 575 mL |
Laboratory supplies
1. 0.2 μm filter (Filtropur BT50, 83.3941.101)
2. 50 mL Screw cap tube (Starstedt, 62.547.254)
3. Microbeads: Silicon dioxide microparticles 2 μm (Sigma-Aldrich, catalog number: 81108-5ML-F), red fluorescent latex beads 2 μm (Sigma-Aldrich, catalog number: L3030-1ML), and non-fluorescent latex beads 3 μm (Sigma-Aldrich, catalog number: LB30-2ML)
Note: Beads are stored at room temperature and covered in foil.
4. 384-well plate (PerkinElmer, PhenoPlate)
5. Hemocytometer (any)
6. “Dummy” plate (any)
7. Pipette tips: 10 μL (VWR, catalog number: 76322-528), 200 μL (VWR, catalog number: 76322-150), and 1000 μL (VWR, catalog number: 76322-154)
8. Pipette: p10 (Eppendorf, catalog number: 3123000020), p200 (Eppendorf, catalog number: 3123000055), and p1000 (Eppendorf, catalog number: 3124000121
9. 1.5 mL microcentrifuge tubes (Avantar, catalog number: 20170-038)
10. Tissue culture plates (Fisherbrand, catalog number: FB012924)
11. Microtube rack (Any)
Equipment
1. Multi-gas incubator (PHCBI, catalog number: MCO-170M-PA)
2. Evos M7000 microscope (ThermoFisher, catalog number: AMF7000)
a. EVOS onstage incubator (Invitrogen, catalog number: AMC1000)
b. EVOS objective lens (20× Air, Plan fluorite 20×/NA 0.5, catalog number: AMEP4698)
c. EVOS light cube (Cy5, catalog number: AMEP4956)
3. Laboratory plate centrifuge (Eppendorf, model: Centrifuge 5810R)
4. Biological safety cabinet (Microzone corporation, model: BK-2-4)
Software and datasets
1. Python (3.10.9)
2. EVOS imaging software (2.1.677.717)
3. GraphPad Prism (10.2.3)
4. KNIME (5.2.1)
5. System requirements:
a. Linux: 64-bit, at least 8 GB RAM
b. Mac: 10.11 and above, at least 8 GB RAM
c. Windows: 32- or 64-bit, at least 8 GB RAM
Procedure
文章信息
稿件历史记录
提交日期: Nov 21, 2024
接收日期: May 26, 2025
在线发布日期: Jul 11, 2025
出版日期: Jul 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Gibson, I., Osterlund, E. J. and Truant, R. (2025). Using Beads as a Focus Fiduciary to Aid Software-Based Autofocus Accuracy in Microscopy. Bio-protocol 15(14): e5388. DOI: 10.21769/BioProtoc.5388.
分类
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











