- EN - English
- CN - 中文
Inducible HIV-1 Reservoir Reduction Assay (HIVRRA), a Fast and Sensitive Assay to Test Cytotoxicity and Potency of Cure Strategies to Reduce the Replication-Competent HIV-1 Reservoir in Ex Vivo PBMCs
诱导型HIV-1库削减检测(HIVRRA):用于评估外周血单个核细胞中HIV-1潜伏库清除策略毒性与效力的快速敏感方法
发布: 2025年07月20日第15卷第14期 DOI: 10.21769/BioProtoc.5384 浏览次数: 2460
评审: Emilie BesnardAnonymous reviewer(s)
Abstract
The HIV-1 reservoir, consisting of transcriptionally silent integrated HIV-1 proviruses, is a major barrier to a cure, as it persists during effective antiretroviral therapy (ART) and is the source of viral rebound upon treatment interruption. Some of the strategies explored for HIV cure focus on the identification of compounds to either reactivate and eliminate the HIV reservoir (“shock and kill”) or to prevent HIV reservoir reactivation and induce deep proviral latency (“block and lock”). Paramount in developing these HIV-1 cure strategies is determining the effect of the compounds on the size of the inducible HIV-1 reservoir in blood from people living with HIV-1 (PWH). Traditionally, viral outgrowth assays have been the primary method to determine the inducible HIV-1 reservoir in CD4+ T cells from PWH. However, these assays are labor-intensive, time-consuming, and often have low sensitivity. We have recently developed the inducible HIV-1 reservoir reduction assay (HIVRRA), a rapid, cost-effective, and sensitive method to measure the impact of compounds on the inducible replication-competent HIV-1 reservoir in total peripheral blood mononuclear cells (PBMCs) from PWH ex vivo. The HIVRRA simultaneously evaluates the effect of test conditions on the size of the inducible replication-competent HIV-1 reservoir as well as the specificity and toxicity of the test strategy. Using total PBMCs instead of purified CD4+ T cells reduces processing time and resource requirements. This makes the HIVRRA a more practical, scalable tool for evaluating potential HIV-1 cure strategies.
Key features
• The HIVRRA builds on the TZM-BL cell-based assay to quantify the HIV-1 reservoir by Sanyal et al.’s [1] method.
• The HIVRRA uses total PBMCs from PWH to determine infectious units per million cells.
• The HIVRRA requires low PBMC input compared to other reservoir analysis methods.
• The HIVRRA determines the toxicity of the compounds on HIV-1-infected and uninfected cells in the same assay.
Keywords: Inducible HIV-1 reservoir reduction assay (HIVRRA) (诱导型HIV-1库削减检测(HIVRRA))Graphical overview
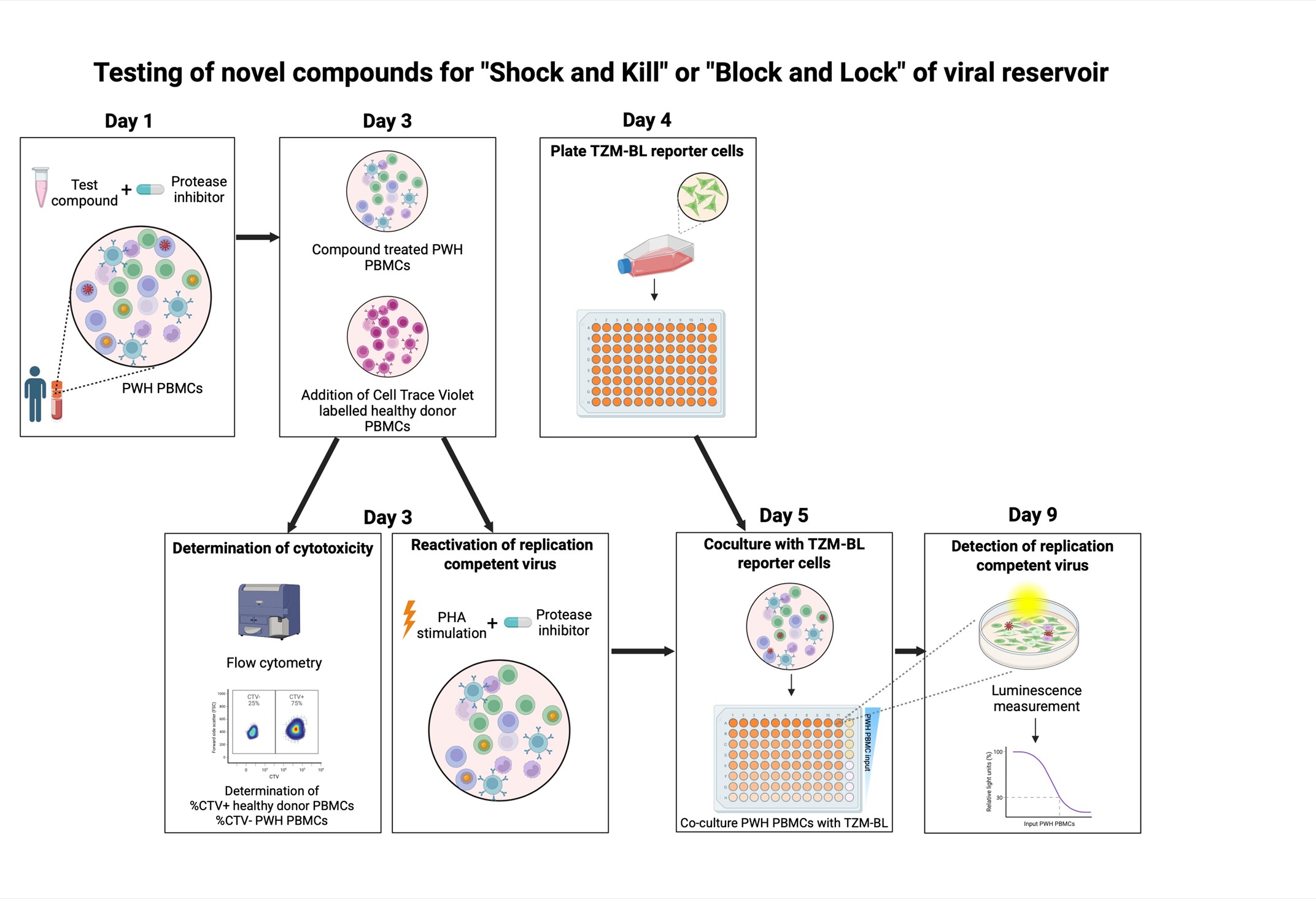
Background
Antiretroviral therapy (ART) has significantly improved the life of people with HIV-1 (PWH) as it effectively suppresses HIV-1 replication. However, ART does not cure HIV-1 and needs to be taken lifelong, since the virus remains present in the reservoir [2]. This HIV-1 reservoir mainly consists of CD4 memory T cells that harbor an integrated provirus in their genome; because the provirus is transcriptionally latent, these cells are not recognized by the immune system [3,4]. Moreover, when ART is interrupted, HIV-1 replication is initiated from the latent viral reservoir [5,6]. This so-called latent but inducible replication-competent HIV-1 reservoir is the source of viral rebound and remains a significant barrier to achieving a cure [3]. Therefore, cure strategies mainly target the replication-competent viral reservoir. One of these strategies is the “shock and kill” method, which aims at eliminating the viral reservoir using latency-reversing agents (LRAs) to reactivate transcription from the latent provirus; this has the goal to eliminate infected cells either through immune recognition or virus- or compound-induced apoptosis [7]. Another method called “block and lock” specifically aims to permanently silence integrated proviruses, through targeting of viral or host factors essential for HIV-1 transcription [8].
The efficacy of LRAs and compounds used for these strategies has been widely tested in in vitro infection and cell line models [9]. However, it is of great importance that the effectiveness of the compounds used for these strategies is also tested in a more relevant setting using peripheral blood mononuclear cells (PBMCs) from people living with HIV-1 (PWH), measuring the inducible replication-competent viral reservoir. Various methods exist to quantify this reservoir. The gold standard for measuring replication-competent virus remains the quantitative viral outgrowth assay (QVOA). The QVOA gives an estimate of the inducible, replication-competent HIV-1 reservoir by serial diluting CD4+ T cells from PWH and stimulating them with activated healthy PBMCs [stimulation occurs through T-cell receptor cross linking using phytohaemagglutinin (PHA) or an antibody cocktail targeting the T-cell receptor (TCR; CD3) and the costimulatory molecule CD28] [10]. It may take up to 4 weeks before a virus grows to detectable levels, and activated PBMCs need to be added to the culture weekly. This makes the QVOA a time-consuming and labor-intensive method with low sensitivity, underestimating the size of the viral reservoir. Moreover, the QVOA requires a large number of cells from PWH, limiting its feasibility for high-throughput screening applications. There is a need for a fast and sensitive assay to measure the impact of interventions on the inducible HIV-1 reservoir.
Sanyal et al [1] developed the TZM-BL cell-based assay (TZA), an alternative approach to quantify the latent HIV-1 reservoir in blood. In this assay, CD4+ T cells from PWH are isolated, activated, and eventually co-cultured with the TZM-BL reporter cell line. This HeLa-derived cell line can be infected by HIV-1 because it has been modified to express the HIV-1 receptor CD4 and coreceptors CXCR4 and CCR5. Furthermore, it contains the β-galactosidase and firefly luciferase reporter genes under the control of the HIV-1 long terminal repeat (LTR). The LTR contains HIV-1 promoter regions that are activated by the HIV-1 protein TAT, which is produced upon infection with HIV-1 [11]. We modified this assay to utilize PBMCs from PWH as input cells and optimized it to test the effect of different compounds on the inducible viral reservoir, simultaneously assessing cytotoxicity. This inducible HIV-1 reservoir reduction assay (HIVRRA) evaluates the ability of compounds of interest to reduce the replication-competent viral reservoir. To this extent, PBMCs from PWH are incubated with compounds. Subsequently, the treated PBMCs are activated through TCR crosslinking to activate viral transcription of the (remaining) inducible viral reservoir. To prevent viral spread during these steps, a protease inhibitor (like ritonavir, indinavir, or saquinavir) is added during compound incubation and TCR crosslinking. As TCR-stimulation is expected to reactivate a large part of the inducible viral reservoir, our assay is suitable to measure a reduction of the inducible viral reservoir. Compounds that either activate or block viral transcription can be used in our assay and will both result in a reduction of the inducible replication-competent viral reservoir, since transcriptional activators like LRAs will initiate HIV-1-related apoptosis, whereas transcriptional blockers will prevent viral replication. Our HIVRRA method is a rapid, cost-effective, and sensitive method for evaluating the impact of compounds on the inducible replication-competent HIV-1 reservoir.
Materials and reagents
Biological materials
1. TZM-BL (NIH AIDS Reagent program, catalog number: 8129); 2 × 106 cells are required for each condition (compounds or untreated) tested
2. PBMCs from uninfected donors (obtained from blood donors from the Dutch national blood bank in Amsterdam, the Netherlands); 8.2 × 105 cells are required for each condition (compounds or untreated) tested
3. PBMCs from PWH on ART (obtained from Amsterdam Cohort Studies on HIV infection and AIDS); 8.2 × 105 cells are required for each condition (compounds or untreated) tested
Reagents
1. Newborn calf serum (NBCS) (ThermoFisher Scientific, catalog number: 16010159)
2. FetalClone II serum (FCS) Cytiva HyCloneTM (Fisher Scientific, catalog number: 10326762)
3. Iscove’s modified Dulbecco’s medium (IMDM) (ThermoFisher Scientific, catalog number: 21980065)
4. Phosphate-buffered saline (PBS) (ThermoFisher Scientific, catalog number: 10010023)
5. Hydrochloric acid (HCl), 37% (Sigma-Aldrich, catalog number: 258148)
6. Phytohaemagglutinin (PHA) (ThermoFisher Scientific, catalog number: R30852801)
7. Ciprofloxacin (ThermoFisher Scientific, catalog number: 449620250)
8. Penicillin-Streptomycin (Pen Strep) (ThermoFisher Scientific, catalog number: 15140122), 10,000 U/mL penicillin/10,000 μg/mL streptomycin
9. IL-2 (Miltenyi Biotec, catalog number: 130-097-742)
10. Trypan Blue (Bio-Rad, catalog number: 1450003)
11. Protease inhibitor Saquinavir (MedChemExpress, catalog number: HY-17007), 10 mM in DMSO
12. RQ1 RNase-free DNAse (Promega, catalog number: M6101), 1,000 U/mL
13. CellTraceTM Violet (ThermoFisher Scientific, catalog number: C34557)
14. Fluorofix (BioLegend, catalog number: 422101)
15. Glycerol (Sigma-Aldrich, catalog number: 818709)
16. Triton X-100 (Fisher Scientific, catalog number: 10509020)
17. Dithiothreitol (DTT) (Duchefa Biochemie, catalog number: D1309)
18. D-Luciferin (Duchefa Biochemie, catalog number: L1349)
19. Na2H2P2O7 (Sigma-Aldrich, catalog number: 71501)
20. MgCl2 (Sigma-Aldrich, catalog number: 814733)
21. ATP (Sigma-Aldrich, catalog number: A2383-1G)
22. NaOH (Sigma-Aldrich, catalog number: 221465)
23. Tris-H3PO4 (Trizma® phosphate monobasic) (Sigma-Aldrich, catalog number: 93348)
24. DMSO (Sigma-Aldrich, catalog number: 102952)
Solutions
1. PHA stock solution (see Recipes)
2. HCl 0.1% (see Recipes)
3. Ciprofloxacin stock solution (see Recipes)
4. IL-2 stock solution (see Recipes)
5. Thaw medium (see Recipes)
6. Cell line medium (see Recipes)
7. Complete medium (see Recipes)
8. Complete medium with protease inhibitor (see Recipes)
9. Complete medium with protease inhibitor and DNAse (see Recipes)
10. PHA medium with protease inhibitor (see Recipes)
11. NaOH (10 M) (see Recipes)
12. Tris-H3PO4 pH 7.8 (1 M) (see Recipes)
13. Triton X-100 (25%)
14. DTT (1 M) (see Recipes)
15. MgCl2 (1 M) (see Recipes)
16. Disodium pyrophosphate (Na2H2P2O7) (50 mM) (see Recipes)
17. ATP (50 mM) (see Recipes)
18. Pre-LAR (see Recipes)
19. Pre-LAR + D-Luciferin (see Recipes)
20. LAR substrate (see Recipes)
Recipes
1. PHA stock solution
Dissolve 2 mg of lyophilized PHA in 10 mL of IMDM at a final concentration of 200 μg/mL. Aliquot and store at -20 °C.
2. HCl 0.1%
Add 2.7 mL of HCl 37% to 1,000 mL of MilliQ H2O. Store at room temperature (RT).
Caution: HCl is a corrosive acid. Handle with care inside a fume hood and use proper personal protection like gloves.
3. Ciprofloxacin stock solution
Dissolve 5 mg of Ciprofloxacin in 2.5 mL of 0.1% HCl (Recipe 2) to a final concentration of 2 mg/mL. Aliquot and store at -20 °C.
4. IL-2 stock solution
Dissolve 30,000 IU recombinant human IL-2 in 15 mL of IMDM to a final concentration of 2,000 U/mL. Aliquot and store at -20 °C.
5. Thaw medium
400 mL of IMDM medium and 100 mL of 20% NBCS. Store and keep at 4 °C before use.
6. Cell line medium
450 mL of IMDM medium, 50 mL of 10% FCS, 5 mL of 100 U/mL penicillin/100 μg/mL streptomycin, and 1.25 mL of 5 μg/mL ciprofloxacin (see Recipe 3). Store at 4 °C.
7. Complete medium
450 mL of IMDM medium, 50 mL of 10% FCS, 5 mL of 100 U/mL penicillin/100 μg/mL streptomycin, 1.25 mL of 5 μg/mL ciprofloxacin (see Recipe 3), and 0.5 mL of 20 IU/mL IL-2 (see Recipe 4). Store at 4 °C.
8. Complete medium with protease inhibitor
Complete medium (see Recipe 7) supplemented with 0.5 mL of 10 µM Saquinavir. Prepare fresh and mix before use.
9. Complete medium with protease inhibitor and DNAse
Add 25 μL of DNase to 10 mL of complete medium (see Recipe 8). Prepare fresh and mix before use.
10. PHA medium with protease inhibitor
Complete medium with protease inhibitor (see Recipe 8) supplemented with 2.5 mL of 1 μg/mL PHA (see Recipe 1). Store at 4 °C. Mix before use.
11. NaOH (10 M)
Weigh 40 g of NaOH and dissolve in small quantities in 150 mL of MilliQ H2O. Add MilliQ H2O up to a final volume of 250 mL. Store at RT.
Caution: Dissolving NaOH generates heat; therefore, NaOH is dissolved in small quantities to obtain the final concentration. 10 M NaOH is a corrosive acid. Handle with care and use proper personal protection like gloves.
12. Tris-H3PO4 pH 7.8 (1 M)
Dissolve 21.9 g of Trizma phosphate monobasic in 60 mL of MilliQ H2O. Adjust pH to 7.8 using 10 M NaOH. Add up to a final volume of 100 mL with MilliQ H2O. Store at RT.
13. Triton X-100 (25%)
Dilute 25 mL of Triton X-100 with 75 mL of MilliQ H2O. Store at RT.
14. DTT (1 M)
Dissolve 154.2 mg of DTT in 0.5 mL of MilliQ H2O. Store at -20 °C.
15. MgCl2 (1 M)
Dissolve 9.522 g of MgCl2 in 80 mL of MilliQ H2O. Add up to a final volume of 100 mL with MilliQ H2O. Store at RT.
16. Disodium pyrophosphate (Na2H2P2O7) (50 mM)
Dissolve 1.1097 g of disodium pyrophosphate in 100 mL of MilliQ H2O. Store solution at RT.
17. ATP (50 mM)
Dissolve 275.6 mg of ATP in 10 mL of MilliQ H2O. Store solution as aliquots of 250 μL at -20 °C. For single use only.
18. Pre-LAR (recipe for 50 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris-H3PO4 (pH 7.8) (1 M) (see Recipe 12) | 38.9 mM | 2.25 mL |
| 100% glycerol | 0.39% | 22.5 mL |
| 25% Triton X-100 (see Recipe 13) | 0.03% | 6 mL |
| DTT (1 M) (see Recipe 14) | 2.6 μM | 50 μL |
| MilliQ H2O | 19.2 mL | |
| Total | 50 mL |
19. Pre-LAR + D-Luciferin (recipe for 50 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Pre-LAR | 50 mL | |
| D-Luciferin | 0.83 mM | 33.33 mg |
| Total | 50 mL |
20. LAR substrate (per plate)
| Reagent | Final concentration | Volume for one 96-well plate |
|---|---|---|
| Pre-LAR | 1.6 mL | |
| MgCl2 (1 M) (see Recipe 15) | 18.7 mM | 56 μL |
| MilliQ H2O | 325 μL | |
| Na2H2P2O7 (50 mM) (see Recipe 16) | 0.78 µM | 4.67 μL |
| ATP (50 mM) (see Recipe 17) | 0.83 mM | 50 μL |
| Pre-LAR + D-Luciferin | 1 mL |
Laboratory supplies
1. White 96-well plates (Thermo Scientific, catalog number: 136101)
2. 96-V bottom plates (GreinerBio, catalog number: 651101)
3. 24-well culture plates (GreinerBio, catalog number: 662160)
4. T75 vented culture flasks (Sarstedt, catalog number: 83.3911.002)
5. 50 mL polypropylene tubes (Corning, catalog number: 430829)
6. 15 mL polypropylene tubes (Corning, VWR, catalog number: 734-0451)
7. Stripettes 5 mL, 10 mL, 25 mL (Corning, catalog numbers: 4487, 4488, 4489)
8. Dual-chamber slides for TC20 automated cell counter (Bio-Rad, catalog number: 1450003). Alternative: Burker-Turk counting chambers (VWR, catalog number: BRND719505)
9. Pipette tips 20–200–1,000 μL (Westburg, catalog numbers: WB 5020L, WB 5040L, WB 5074S)
10. 1.5 mL or 2 mL Eppendorf tube (sterile) (VWR, catalog numbers: 211-2130, 211-2120)
Equipment
1. Flow cytometer (BD Biosciences, model: BD FACSCantoTM)
2. Luminometer (Berthold Technologies, catalog number: 1015-277R1, model: LB942 Tristar2)
3. TC20 automated cell counter (Bio-Rad, catalog number: 1450102)
4. Incubator (Thermo Fisher, model: Thermo Forma Direct Heat CO2 incubator type 321)
5. Pipettes (Gilson, p20, p200, p100; Eppendorf p10)
6. Multichannel pipette, 12 channels for 5–50 μL and 30–300 μL (Thermo Scientific catalog numbers: 4661050N and 4661070N)
7. Light microscope (Leica, model: DM IL HC Bio Microscope with table, catalog number: 11521227)
8. Water bath (Fisher Scientific Emergo, model: Schudwaterbad SW-22 catnr: 608044)
9. Pipette boy (Life Technologies Europe BV, catalog number: 9521, model: S1 Pipet Fillers)
10. Centrifuge for Eppendorf tubes (Eppendorf, model: centrifuge 5417R 230V “Refresher”)
11. Centrifuge for 15 and 50 mL tubes (Hettich, model: ROTANTA/460 RS)
Software and datasets
1. BD FACSDivaTM software (BD Biosciences) (license needed)
2. FlowJo software (Tree Star, version 10) (license needed)
3. Microsoft Excel (Microsoft Corporation, version 2016, release date: 09/22/15) (license needed)
4. GraphPad Prism (GraphPad Software Inc., version 10, release date: 07/11/23) (license needed)
Procedure
文章信息
稿件历史记录
提交日期: Apr 18, 2025
接收日期: Jun 16, 2025
在线发布日期: Jul 4, 2025
出版日期: Jul 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Jansen, J., Geijtenbeek, T. B.H. and Kootstra, N. A. (2025). Inducible HIV-1 Reservoir Reduction Assay (HIVRRA), a Fast and Sensitive Assay to Test Cytotoxicity and Potency of Cure Strategies to Reduce the Replication-Competent HIV-1 Reservoir in Ex Vivo PBMCs. Bio-protocol 15(14): e5384. DOI: 10.21769/BioProtoc.5384.
分类
微生物学 > 病原体检测
细胞生物学 > 基于细胞的分析方法 > 病毒性感染
微生物学 > 微生物-宿主相互作用 > 病毒
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












