- EN - English
- CN - 中文
Multi-phase Training in Virus-Like Particle Synthesis to Foster Science Self-efficacy in Students With Minimal Laboratory Experience
面向低实验基础学生的多阶段病毒样颗粒合成训练对科学自我效能的培养
(*contributed equally to this work) 发布: 2025年07月20日第15卷第14期 DOI: 10.21769/BioProtoc.5381 浏览次数: 1749
评审: David Paul
Abstract
Science self-efficacy describes the confidence individuals have in their ability to accomplish specific scientific practices. Self-efficacy is one factor linked to success and persistence within STEM fields. The purpose of this protocol is to provide research laboratories with effective methods for teaching and mentoring new students in molecular biology, specifically in the synthesis of virus-like particles (VLPs) derived from bacteriophages. VLPs are multivalent nanoparticle structures that can be utilized in multiple biomedical applications, including platforms for vaccine and drug delivery. Production of bacteriophage VLPs using bacterial expression systems is feasible in most laboratory settings. However, synthesizing and characterizing VLPs can be challenging for new researchers, especially those with minimal laboratory experience or a lack of foundational knowledge in molecular biology. To address this, a multi-phase training protocol was implemented to train new students in VLP synthesis, purification, and characterization. This model was optimized for training numerous high school and undergraduate students. By implementing this multi-phase methodology, the students’ confidence in their abilities to perform specific tasks increased and likely enhanced their persistence in STEM.
Key features
• Multi-phase training model for new students.
• Training phases that build to increase science self-efficacy.
• Successful peer-to-peer training.
Keywords: Peer-to-peer training (同伴互助培训)Graphical overview
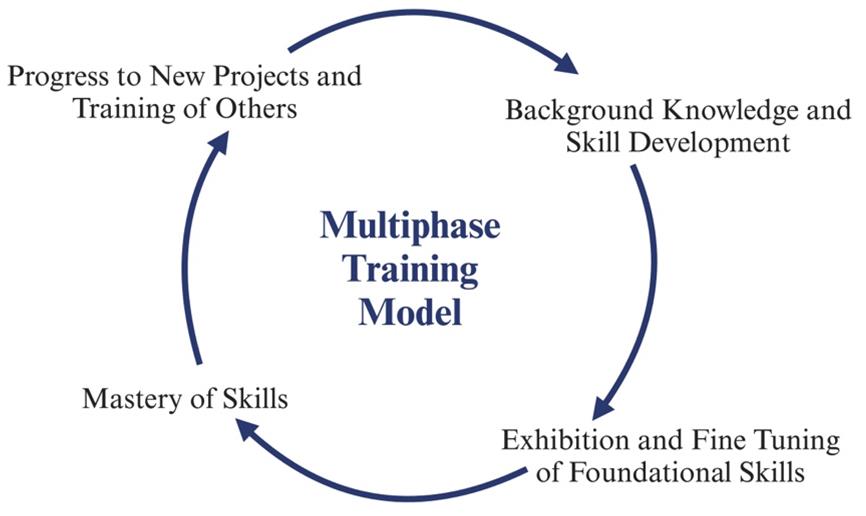
Multiphase training model: The three-phase process can train students on fundamental molecular biology skills. Phase I focuses on preparing students for the project by reviewing an individual development plan (IDP) with their mentor, reviewing protocols established by the lab, and reading relevant articles. The first phase concludes with students observing their peers on the protocols and asking questions for clarity. Phase II involves the students conducting hands-on experiments with guidance from a peer. Students may ask for clarity on protocols and mistakes from their peer or supervisor. Students also become familiar with lab-related software. Students transition into phase III when they are confident to work independently. The scale of the experiments also increases with self-efficacy. By the end of Phase II, students can develop an abstract and a poster related to their project.
Background
Science self-efficacy is an individual’s confidence in their ability to complete specific scientific practices such as research, grant writing, or development of protocols [1]. Self-efficacy is one factor linked to success and persistence within STEM fields among aspiring scientists, especially racial/ethnic minority students [2]. It is important for students with minimal laboratory experience to build their science self-efficacy to increase their likelihood of persistence in STEM. We describe a multi-phase model designed to build students' confidence within each phase, adding to their overall science self-efficacy. This model is implemented among high school and undergraduate students with little or no laboratory experience.
Phase I is focused on building strong foundations through the fundamentals of research (Graphical overview). Once students have a fundamental understanding of the research they are working on, they progress to Phase II. Within Phase II, students work on developing their skills through hands-on learning experiences, allowing them to familiarize themselves with instruments utilized for their research. Therefore, it is not uncommon for students to make mistakes during the learning process. Students can learn from making mistakes and recognize how learning from mistakes is a crucial aspect of developing critical thinking and problem-solving skills. Phase II concludes with students demonstrating their ability to produce and purify proteins by growing small bacterial cultures (100 mL) of virus-like particles (VLPs) with high yields and purifying the VLPs by size-exclusion chromatography. Phase III promotes confidence in students' skills, preparing them to lead peer-to-peer training for incoming students, present at conferences, and develop and fine-tune their skills for future research opportunities. This approach fosters scientific fluency and confidence and can be adapted to any type of bench-based research. Phases I and II require at least a semester or 15 weeks of training. Ultimately, students can successfully complete all three phases within one academic year (Figure 1).
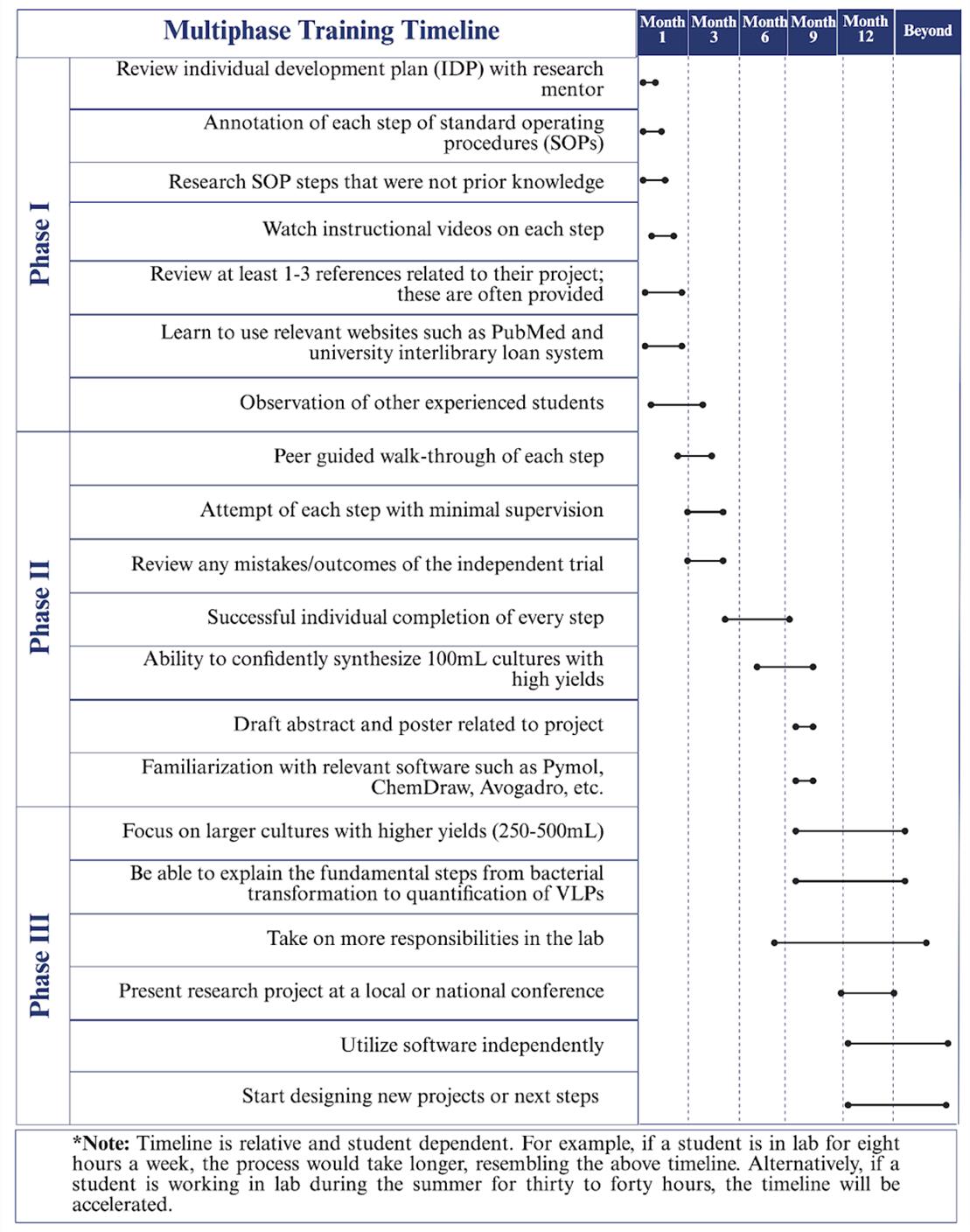
Figure 1. Multiphase training timeline. The three-phase training timeline can take up to one academic year. Phase I focuses on required training such as IDP development, SOP reviews, and instructional guidance review. Phase II relies on peer guidance through the protocol to produce VLPs and to prepare presentations. Finally, Phase III promotes individual completion of the protocol with high production of VLPs and the presentation of their work.
This protocol explains how to implement the multi-phase method using the synthesis and purification of virus-like particles (VLPs) as a model activity. VLPs consist of viral structural proteins that, when overexpressed, spontaneously self-assemble into particles that resemble the virus they are derived from [3]. Importantly, because VLPs do not contain viral genetic material, they are completely non-infectious. VLPs resemble infectious virions and have been used as vaccines for several viruses, including human papillomavirus (HPV) and hepatitis B virus (HBV). Their highly repetitive and multivalent structures render them highly immunogenic, making them suitable as vaccine platforms for targeting diverse molecules, including antigens from pathogens, self-molecules involved in chronic diseases, and even drugs of abuse [3–5]. VLPs have also been used in other biomedical applications, including drug delivery [5]. While VLPs are produced in a variety of expression systems, including mammalian cells, insect cells, and yeast, production of VLPs derived from bacteriophages using an E. coli bacteria-based expression system is accessible to most laboratory settings.
VLPs derived from a family of single-stranded RNA (ssRNA) bacteriophages, including AP205, Qβ, and MS2, have been widely used as vaccine platforms due to their ability to display diverse vaccine targets [6,7]. MS2 VLPs consist of a single structural coat protein that dimerizes and self-assembles into a ~27 nm diameter particle composed of 90 coat protein dimers (Figure 2A). In contrast, Qβ VLPs assemble into pentamers and hexamers, stabilized through disulfide linkages to form a ~30 nm diameter particle made up of 180 coat protein monomers, each with surface-exposed lysines, making up 540 antigen attachment sites (Figure 2B). There are multiple ways to display antigens on the surface of bacteriophage VLPs [6,8,9], with the two most common being genetic engineering and chemical conjugation. However, this manuscript focuses on the synthesis and purification of unmodified VLPs, primarily Qβ, though the protocol can be utilized for various research areas.
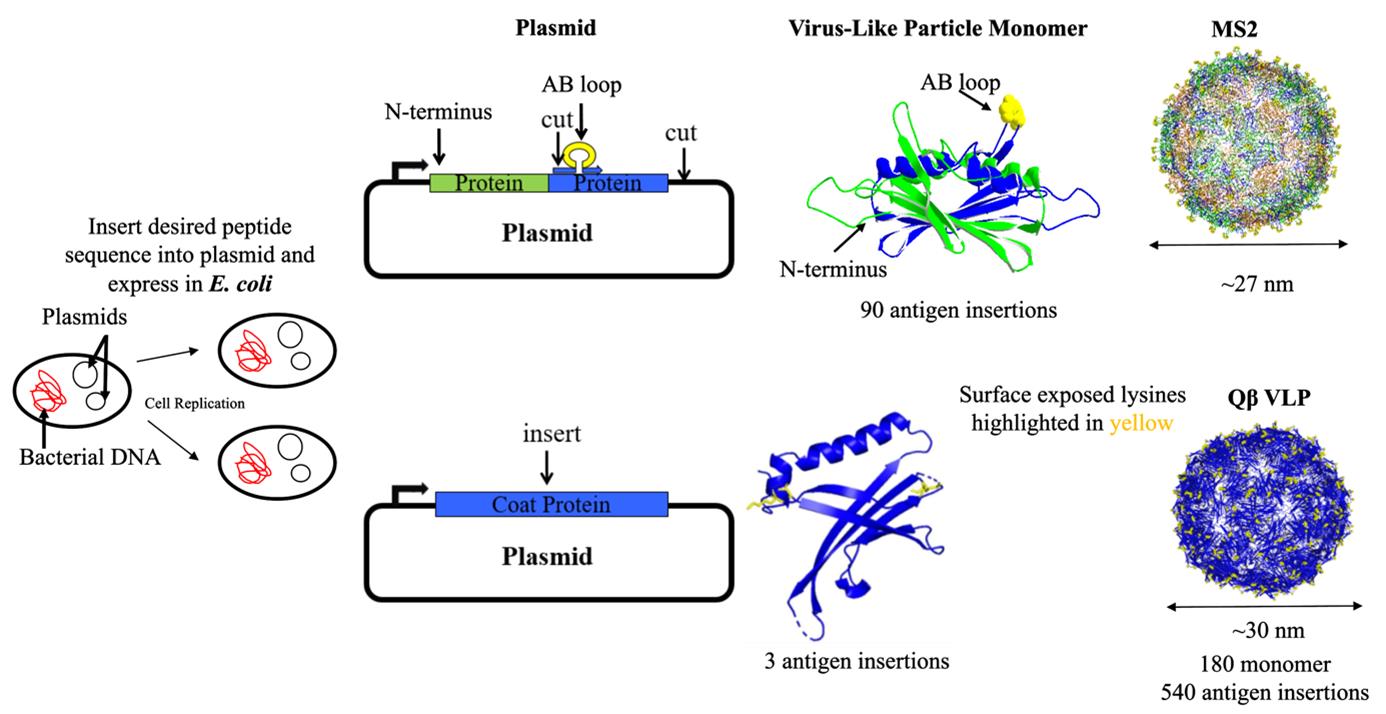
Figure 2. Synthesis pathway of Qβ and MS2 virus-like particles (VLPs). This figure illustrates the engineering of VLP plasmids and their expression in E. coli through bacterial transformation. The MS2 VLP plasmid (top) includes a human papillomavirus (HPV) antigen inserted at the N-terminus of the single-chain dimer of the coat protein. These coat proteins self-assemble into ~27 nm VLPs displaying the HPV antigen, with potentially 90 antigen insertion sites. The Qβ VLP plasmid (bottom) features the insertion of a lysine amino acid into the coat protein. These coat proteins self-assemble into ~30 nm VLPs that display lysines (highlighted in yellow) on their surface with potentially 540 antigen insertion sites.
Materials and reagents
Biological materials
1. OverExpressTM C41(DE3) chemically competent cells (Sigma-Aldrich, catalog number: CMC0017)
2. E. coli 10G ELITE® SixPack Electrocompetent Cells (Biosearch Technology, catalog number: 60052-3)
3. Q-beta and pDSP62 plasmids (obtained from the Chackerian Lab at the University of New Mexico)
Reagents
1. 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M3148)
2. 2-(N-morpholino)ethanesulfonic acid (MES) free acid (Gold Bio, catalog number: M-095-1)
3. Ammonium sulfate (Sigma-Aldrich, catalog number: A4418)
4. Bacto yeast extract (Thermo Fisher Scientific, catalog number: 212750)
5. Bromphenol blue (Sigma-Aldrich, CAS number: 115-39-9)
6. Certified molecular biology agarose (Bio-Rad, catalog number: 1613102)
7. Coomassie blue R-250 (Thermo Fisher Scientific, CAS number: 6104-59-2)
8. DNase 1 (Thermo Fisher Scientific, catalog number: 11284932001)
9. Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) (Sigma-Aldrich, catalog number: E9884)
10. UltraPureTM ethidium bromide, 10 mg/mL (Thermo Fisher Scientific, catalog number: 15585011)
11. Acetic acid, glacial (Sigma-Millipore, catalog number: AX0073-75)
12. Glycerol (Thermo Fisher Scientific, CAS number: 56-81-5)
13. ImMediaTM Kan agar (Thermo Fisher Scientific, catalog number: 45-0043)
14. Isopropyl β-D-thiogalactopyranoside (IPTG) (Thermo Fisher Scientific, catalog number: 15529019)
15. Kanamycin disulfate salt from Streptomyces kanamyceticus (Sigma-Aldrich, catalog number: K1876)
16. Lysozyme from chicken egg white (HEL) (Sigma-Aldrich, catalog number: L6876)
17. Magnesium chloride solution (Sigma-Aldrich, catalog number: 68475)
18. Magnesium sulfate solution (Sigma-Aldrich, catalog number: M3409)
19. Orange G (Sigma-Aldrich, catalog number: O3756)
20. Tris base (Sigma-Aldrich, catalog number: 648310-M)
21. Potassium chloride (Fisherbrand, catalog number: P217-500)
22. Potassium phosphate monobasic (Fisherbrand, catalog number: P285-500)
23. SeeBlueTM Pre-stained standard (1×) (Invitrogen, catalog number: LC5625)
24. Sodium acetate (Sigma-Aldrich, catalog number: S2889)
25. Sodium chloride (Fisherbrand, CAS number: 7647-14-5)
26. Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L3771)
27. Sodium phosphate dibasic (Fisherbrand, CAS number: 7558-79-4)
28. Tris-base (Sigma-Aldrich, catalog number: 648310-M)
29. Tris (1 M, pH 8.4) (Alfa Aesar, CAS number: 77-86-1)
30. Tris-HCl (1 M, pH 7.5) (Fisherbrand, catalog number: BP1757)
31. Tryptone (Sigma-Millipore, catalog number: T9410)
32. Sodium hydroxide (NaOH) pellet 500 G AR® (ACS) (Thomas Scientific, catalog number: C818M3)
Solutions
Note: The “×” unit (i.e., 1×) indicates the concentration of a solution.
1. 5× loading dye (for loading protein onto an acrylamide gel) (see Recipes)
2. 6× orange loading dye (for loading DNA onto an agarose gel) (see Recipes)
3. 1% agarose gel (see Recipes)
4. 10% deoxycholate solution (DOC) (see Recipes)
5. 70% ethanol solution (see Recipes)
6. 1× 2-(N-Morpholino)-ethanesulfonic acid buffer (MES) (see Recipes)
7. 1× phosphate-buffered saline (PBS) (see Recipes)
8. 1× SCB (see Recipes)
9. 1× tris-acetate EDTA (TAE) (see Recipes)
10. 0.5 M ethylenediaminetetraacetic acid disodium salt dihydrate solution (EDTA) (see Recipes)
11. 5 M sodium chloride solution (see Recipes)
12. Coomassie blue low-toxicity staining solution (for staining acrylamide gel) (see Recipes)
13. 10 mg/mL DNase solution (see Recipes)
14. 100 mg/mL kanamycin solution (see Recipes)
15. Isopropyl β-D-thiogalactopyranoside solution (IPTG) (see Recipes)
16. Lysogeny broth (LB) (see Recipes)
Recipes
diH2O = deionized water
dH2O = distilled water
UPW = ultrapure water
1. 5× loading dye
Note: For loading protein onto an SDS PAGE gel electrophoresis. It contains SDS, which is an irritant. Avoid inhalation and skin contact.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-HCl (1 M, pH 6.8) | 0.2 g/L | 2 mL |
| Sodium dodecyl sulfate | 277 mM | 0.8 g |
| Glycerol | 4.90 M | 4 mL |
| β-mercaptoethanol | 570 mM | 0.4 mL |
| Bromophenol blue | 1.2 mM | 8 mg |
| diH2O | n/a | 3.6 mL |
| Total | n/a | 10 mL |
a. Measure all the reagents and diH2O into a 100 mL graduated glass cylinder.
b. Mix vigorously using a vortex mixer to dissolve reagents.
c. Aliquot into 1.5 mL microcentrifuge tubes.
d. Store at -20 °C. Stable for one year under these conditions.
2. 6× Orange DNA loading dye
Note: For loading DNA onto an agarose gel electrophoresis.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| TAE (50×) | 3.10 M | 2 mL |
| Orange G | 0.033 M | 0.15 g |
| Glycerol | 0.00407 M | 60 mL |
| dH2O | n/a | 38 mL |
| Total | 3.13 M | 100 mL |
a. Measure all the reagents and diH2O into a 100 mL graduated glass cylinder.
b. Mix vigorously using a vortex mixer to dissolve reagents.
c. Aliquot into 1.5 mL microcentrifuge tubes.
d. Store at room temperature. Stable for one year under these conditions.
3. 1% agarose gel (100 mL)
Note: When handling ethidium bromide, open the bottle facing AWAY from you to avoid eye/skin contact. When heating/cooling the solution, avoid inhaling the steam. Ethidium bromide is highly toxic and an irritant.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| TAE (1×) | n/a | 100 mL |
| Agarose powder | n/a | 1 g |
| Ethidium bromide | 0.0001 mg/mL | 1 µL |
| Total | 1 g/100 mL | 100 mL |
a. Measure all the reagents, except ethidium bromide, into a 200 mL glass Erlenmeyer flask.
b. Microwave for 1 min 30 s.
c. Cool solution in a fume hood until warm to the touch.
d. Add ethidium bromide.
e. Pour into a gel mold to let it cool in a 4 °C fridge until it settles.
4. 10% deoxycholate solution (DOC) (100 mL)
Note: Solution decomposes when exposed to ‘direct light. Perform in a room with minimal lighting.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium deoxycholate | 10 g/100 mL | 10 g |
| dH2O | n/a | 100 mL |
| Total | 10 g/100 mL | 100 mL |
a. Wrap the bottle with aluminum foil, neck to bottom, to avoid any light contact.
b. Place a magnetic stir bar in the bottle.
c. Add 80 mL of dH2O and 10 g of sodium deoxycholate.
d. Immediately close the lid and stir the solution on a magnetic stir plate.
e. Let it stir for 10–15 min.
f. Store at room temperature. Stable for one year under these conditions.
5. 70% ethanol solution (100 mL)
Note: Pure ethanol is highly flammable and an irritant. Avoid handling near flames and direct contact with the skin.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ethanol (100%) | 70% | 70 |
| dH2O | n/a | 30 mL |
| Total | 70 g/100 mL | 100 mL |
a. Measure all the reagents and dH2O into an amber/brown bottle.
b. Invert the bottle a few times to mix the solution.
c. Store at room temperature.
6. Coomassie Blue low-toxicity staining solution
Note: Solution may stain skin and clothing. Wear gloves and a lab coat for precaution.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ethanol (95%) | 0.027 M | 500 mL |
| Coomassie Blue R-250 [0.1% (w/w)] | 0.0012 M | 1.25 g |
| Glacial acetic acid [99.7% (w/w)] | 0.111 M | 125 mL |
| dH2O | n/a | 500 mL |
| Total | 0.139 M | 1,250 mL |
a. Measure Coomassie blue and ethanol in a 2 L graduated glass cylinder.
b. Add 500 mL of diH2O.
c. Dissolve the dye completely by sealing the opening and inverting the glassware to mix.
d. Add glacial acetic acid, seal the opening, and invert the glassware to mix.
e. Bring volume up to a total of 1,250 mL with dH2O and invert to mix for 5 min.
f. Store at room temperature. Stable for one year under this condition.
7. 2-(N-Morpholino) ethanesulfonic acid buffer (1 L)
Note: MES buffer is used for running acrylamide gels (for protein). It contains SDS, which is an irritant. Avoid inhalation and skin contact.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 2-(N-Morpholino) ethanesulfonic acid | 0.04 M | 9.76 g |
| Tris base | 1.0 M | 6.06 g |
| Sodium dodecyl sulfate | 0.0693 M | 1.0 g |
| EDTA | 0.0205 M | 0.3 g |
| diH2O | n/a | 1 L |
| Total | 1.1298 M | 1 L |
a. Measure all the reagents and diH2O into a 1 L Duran bottle.
b. Add 500 mL of diH2O.
c. Dissolve completely by sealing the opening, adding a magnetic stir bar, and letting it stir on a magnetic stirrer.
d. Bring volume up to a final volume of 1 L with diH2O and mix. Remove the stir bar.
e. Store at room temperature. Stable for one year under this condition.
8. Phosphate-buffered saline (PBS) (20 L)
Note: Allow the reagents to dissolve completely in 500 mL of UPW before adding any more UPW. Store at room temperature.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride | 136 mM | 160 g |
| Potassium chloride | 2 mM | 4 g |
| Sodium phosphate dibasic | 8.1 mM | 23 g |
| Potassium phosphate monobasic | 1.46 mM | 4 g |
| Ultrapure water (UPW) | n/a | 20 L |
| Total | 147.56 mM | 20 L |
a. Fill a 20 L jug with UPW.
b. Remove 1 L from the jug into a separate glassware.
c. Measure all the reagents above and add to the 1 L UPW.
d. Dissolve completely by sealing the opening, adding a magnetic stir bar, and letting it stir on a magnetic stirrer.
e. Remove the stir bar and pour the dissolved solution into the 20 L jug.
f. Mix the solution in the jug by rocking it back and forth.
g. Store at room temperature. Stable for one year under this condition.
9. SCB buffer (1 L)
Note: Allow the reagents to dissolve completely in 500 mL of diH2O before adding any more diH2O. Store at 4 °C.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-HCl (1 M, pH 7.4) | 0.01 M | 10 mL |
| Sodium chloride solution (5 M) | 0.1 M | 20 mL |
| Magnesium sulfate solution (1 M) | 0.001 M | 1 mL |
| diH2O | n/a | 969 mL |
| Total | 0.111 M | 1 L |
a. Measure all the reagents into a 1 L Duran bottle.
b. Add 500 mL of diH2O.
c. Dissolve completely by sealing the opening, adding a magnetic stir bar, and letting it stir on a magnetic stirrer.
d. Bring volume up to a final volume of 1 L with diH2O and mix. Remove the stir bar.
e. Store at 4 °C. Stable for one year under this condition.
10. TAE buffer (1 L of 1×)
Note: TAE (Tris-Acetate-EDTA) is the most common buffer used for agarose gel electrophoresis for the analysis of nucleotides or DNA.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-base | 40 mM | 4.844 g |
| Sodium acetate | 5 mM | 0.41 g |
| EDTA | 2 mM | 0.7445 g |
| Glacial acetic acid [99.7% (w/w)] | 33.6 mM | 1.93 mL |
| diH2O | n/a | 1 L |
| Total | 80.6 mM | 1 L |
a. Measure all the reagents and diH2O into a 1 L Duran bottle.
b. Add 500 mL of diH2O.
c. Dissolve completely by sealing the opening, adding a magnetic stir bar, and letting it stir on a magnetic stirrer.
d. Bring volume up to a final volume of 1 L with diH2O and mix. Remove the stir bar.
e. Store at 4 °C. Stable for one year under this condition.
11. 0.5 M EDTA solution (100 mL)
Note: Allow the reagents to dissolve completely in 80 mL of dH2O and measure the pH level before adding any more dH2O. EDTA is an irritant. Avoid skin/eye contact. Wear gloves when handling.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| EDTA | 0.5 M | 18.61 g |
| Sodium hydroxide (NaOH) pellets | 0.5 M | 2 g |
| dH2O | n/a | 100 mL |
| Total | 1.0 M | 100 mL |
a. Measure EDTA into 500 mL Duran bottle.
b. Add 80 mL of dH2O and adjust the pH to 8.0 with NaOH pellets.
Note: EDTA will not dissolve completely into solution until pH 8.0.
c. Once fully dissolved, bring volume up to 100 mL with dH2O, add a magnetic stir bar, and let it stir for 5 min.
d. Store in an acid cabinet at room temperature. Stable for one year under these conditions.
12. 5 M sodium chloride solution (100 mL)
Note: Allow the reagents to dissolve completely in 800 mL of dH2O before adding any more dH2O.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium chloride | 5 M | 292 g |
| dH2O | n/a | 1 L |
| Total | 5 M | 1 L |
a. Measure sodium chloride and 800 mL of dH2O in a 1 L Duran bottle.
b. Dissolve completely by sealing the opening, adding a magnetic stir bar, and letting it stir on a magnetic stirrer.
c. Once fully dissolved, bring volume up to 1 L with dH2O and let it stir for 5 min. Remove the stir bar.
d. Store at room temperature. Stable for one year under this condition.
13. DNase solution (10 mL of 10 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DNase 1 | 10 mg/mL | 100 mg |
| Nuclease-free H2O | n/a | 10 mL |
| Total | 10 mg/mL | 10 mL |
a. Measure DNase 1 and nuclease-free H2O in 100 mL glassware.
b. Stir to mix until dissolved.
c. Aliquot in 1.5 mL microcentrifuge tubes.
d. Store at -20 °C. Stable for one year under this condition.
14. IPTG solution (20 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| IPTG | 23.8 mg/mL | 0.476 g |
| diH2O | n/a | 20 mL |
| Total | 23.8 mg/mL | 20 mL |
a. Measure IPTG and diH2O in 100 mL glassware.
b. Stir to mix until dissolved.
c. Sterilize the solution by filtering through a microcentrifuge spin column.
d. Centrifuge for 1 min at 9,021.68 rcf.
e. Collect filtrate and aliquot in 1.5 mL microcentrifuge tubes.
f. Store at -20 °C. Stable for one year under this condition.
15. Kanamycin solution (100 mL)
Note: Kanamycin is a health hazard. Avoid direct skin contact and inhalation. Wear gloves when handling.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Kanamycin | 100 mg/mL | 1 g |
| dH2O | n/a | 10 mL |
| Total | 100 mg/mL | 10 mL |
a. Measure kanamycin and dH2O in 100 mL glassware.
b. Stir to mix until dissolved.
c. Centrifuge for 1 min at 9,021.68 rcf.
d. Collect filtrate and aliquot in 1.5 mL microcentrifuge tubes.
e. Store at -20 °C. Stable for one year under this condition.
Media
Most of the following media should have a final pH of 7.0 ± 0.2, as E. coli grows best in a neutral solution. Generally, the role of a base in a growth medium is to bring the solution to a neutral or nearly neutral pH. When no other base is mentioned, use NaOH as necessary.
When doubling ingredients for a medium, but not the final volume, to make a concentrated, rich growth medium (such as 2× LB, a doubly concentrated form of LB), unless otherwise instructed, one should not double the acids or bases, except when necessary for adjusting pH level. Also, one should generally not double the salt.
Media should be aliquoted and autoclaved shortly after being made. If it cannot be autoclaved within a reasonable time (before the end of the day), it should be refrigerated to prevent the growth of contaminants.
1. LB broth (1 L)
Note: LB (an acronym for lysogeny broth, although it is often misinterpreted as Luria Broth, Lennox Broth, or Luria-Bertani medium) was originally developed to optimize Shigella growth and plaque formation. It is the standard growth medium for Escherichia coli (a relative of Shigella).
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tryptone | 140 mM | 10 g |
| Yeast extract | n/a | 5 g |
| Sodium chloride | 85.5 mM | 5 g |
| diH2O | n/a | 1 L |
| Total | 225.5 mM | 1 L |
a. Measure all the reagents into a 1 L glassware.
b. Add 500 mL of diH2O.
c. Dissolve completely by sealing the opening, adding a magnetic stir bar, and letting it stir on a magnetic stirrer.
d. Once fully dissolved, bring volume up to 1 L with dH2O and let it stir for 5 min. Remove the stir bar.
e. To make 100 mL, 250 mL, and 500 mL cultures, pour LB solution into an Erlenmeyer Flask as follows:
100 mL of LB solution in a 250 mL flask
250 mL of LB solution in a 500 mL flask
500 mL of LB solution in a 1,000 mL flask
f. Seal the flask opening with aluminum foil and paste a small strip of autoclave tape on the neck of the flask.
g. Autoclave at 121 °C for 1 h.
h. Once cooled, swirl the flask to ensure it is fully mixed.
i. Store at room temperature. Stable for 2–3 months under this condition.
Laboratory supplies
1. BemisTM ParafilmTM M laboratory wrapping film, 4 in. × 125 ft. (Fisher Scientific, catalog number: 13-374-10)
2. Celltreat round bottom centrifuge tube, 50 mL (Fisher Scientific, catalog number: 50-828-732)
3. CryoKING premium cardbox freezer boxes, 3 in., 81 wells (BiologixUSA, catalog number: 90-2381)
4. Culture tubes with closer, 17 × 100 mm, vol. 14 mL (VWR, catalog number: 60818-664)
5. Dialysis tub (VPET Plastics, catalog number: J110CT-128-02)
6. Easy ReaderTM conical polypropylene centrifuge tubes, 15 mL (Fisher Scientific, catalog number: 05-539-4)
7. Easy ReaderTM conical polypropylene centrifuge tubes, 50 mL (Fisher Scientific, catalog number: 339653)
8. GeneMate graduated microcentrifuge tubes, 1.7 mL (VWR, catalog number: 490004-436)
9. InvitrogenTM gel knife (Thermo Fisher Scientific, catalog number: EI9010)
10. Gene Pulsers/MicroPulserTM cuvettes, 0.1 cm gap (Bio-Rad, catalog number: 165-2089)
11. Nichrome/aluminum inoculating loops (Fisher Scientific, catalog number: 13-104-5)
12. Nonpyrogenic serological pipette, 5 mL (Fisher Scientific, catalog number: 13-678-11D)
13. Nonpyrogenic serological pipette, 10 mL (Fisher Scientific, catalog number: 13-678-11E)
14. Nonpyrogenic serological pipette, 25 mL (Fisher Scientific, catalog number: 13-678-11)
15. NuPAGETM 10%, Bis-Tris, 1.0–1.5 mm, mini protein gels (Thermo Fisher Scientific, catalog number: NP0302BOX)
16. PierceTM protein concentrator PES, 100 MWCO, ≤ 0.5 mL (Thermo Fisher Scientific, catalog number: 88503)
17. PierceTM protein concentrator PES, 100 MWCO, 2–6 mL (Thermo Fisher Scientific, catalog number: 88523)
18. PierceTM protein concentrator PES, 100 MWCO, 5–20 mL (Thermo Fisher Scientific, catalog number: 88533)
19. Petri dish, slippable lid 100 mm × 15 mm, sterile, polystyrene (Fisherbrand, catalog number: FB0875713)
20. Plastic cuvettes, 1–1.5 mL (UV-VIS) (Vernier, catalog number: cuv-uv)
21. SnakeskinTM dialysis tubing (Thermo Fisher Scientific, catalog number: 68035)
22. SnakeskinTM dialysis tubing clips (Thermo Fisher Scientific, catalog number: 68011)
23. TipOne pipette tips, 10 μL (USA Scientific, catalog number: 1111-3000)
24. TipOne pipette tips, 300 μL (USA Scientific, catalog number: 1110-9000)
25. TipOne pipette tips, 1,250 μL (USA Scientific, catalog number: 1112-1020)
26. Octagonal stirring bar kit PTFE (Dynalon Labware, catalog number: 303595)
Equipment
1. BarnsteadTM GenpureTM Pro water purification system (Thermo Fisher Scientific, catalog number: 50131948)
2. Biowave cell density meter CO8000 (Avantor, model: WPA CO8000, catalog number: 80-3000-45)
3. Branson UltrasonicTM SonifierTM SFX250 cell disruptors (Fisher Scientific, catalog number: 15-345-138)
4. Compact digital rocker, 100–240 V, 50/60 Hz, US plug (Fisher Scientific, catalog number: 88880019)
5. Digital dry bath (Bio-Rad, catalog number: 1660562EDU)
6. FisherBiotech transilluminator (Fisher Scientific, model: DLT)
7. Xcell SurelockTM mini-cell (Invitrogen, catalog number: EI0001)
8. Magnetic stirrer (ANZESER, catalog number: B07J59QVGQ)
9. MaxQ 4000 incubated shaker (Thermo Fisher Scientific, model: SHKE4000-7)
10. MicroPulserTM (Bio-Rad, model: 411BR 12141)
11. Mini-Sub® cell GT cell (Bio-Rad, model: Mini-Sub® Cell GT)
12. Model 2110 fraction collector (Bio-Rad, catalog number: 7318122)
13. mySPINTM 12 mini centrifuge (Thermo Fisher Scientific, catalog number: 75004081)
14. MYTEMPTM mini digital incubator (Benchmark Scientific, model: H2200-H)
15. Ohaus AdventurerTM analytical balance (Ohaus, catalog number: AR1140)
16. PowerPacTM basic power supply (Bio-Rad, catalog number: 1645050)
17. RevcoTM UxF -86 °C upright ultra-low temperature freezers (Fisher Scientific, model: UXF60086D)
18. Sorvall legend XTR centrifuge (Thermo Fisher Scientific, catalog number: 75004521)
19. Sorvall LYNX 4000 superspeed centrifuge (Thermo Fisher Scientific, catalog number: 75006580)
20. Variable speed vortex mixer (Fisher Scientific, catalog number: 14-955-163)
21. Wide Mini-Sub® cell GT cell (Bio-Rad, model: Wide Mini-Sub® Cell GT)
Software and datasets
1. EnCor Biotechnology Inc., ammonium sulfate calculator: https://www.encorbio.com/protocols/AM-SO4.htm (access date, 9/10/2023)
Procedure
文章信息
稿件历史记录
提交日期: Sep 18, 2024
接收日期: Jun 3, 2025
在线发布日期: Jul 11, 2025
出版日期: Jul 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Proctor-Roser, M. A., Faimau, M., Peabody, J., Charley, K. R., Chackerian, B. and Lee, N. R. (2025). Multi-phase Training in Virus-Like Particle Synthesis to Foster Science Self-efficacy in Students With Minimal Laboratory Experience. Bio-protocol 15(14): e5381. DOI: 10.21769/BioProtoc.5381.
分类
分子生物学 > 蛋白质 > 表达
微生物学 > 异源表达系统
生物化学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













