- EN - English
- CN - 中文
Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
基于天然纤维蛋白生物墨水的三维共培养皮肤模型构建及其感染模型实验方案
发布: 2025年07月20日第15卷第14期 DOI: 10.21769/BioProtoc.5380 浏览次数: 3788
评审: Samantha HallerAnonymous reviewer(s)
Abstract
The skin microbiome, a diverse community of microorganisms, plays a crucial role in maintaining skin health and homeostasis. Traditional studies have relied on two-dimensional (2D) models, which fail to recreate the complex three-dimensional (3D) architecture and cellular interactions of in vivo human skin, and animal models, which have species-specific physiology and accompanying ethical concerns. Consequently, both types of models fall short in accurately replicating skin physiology and understanding its complex microbial interactions. Three-dimensional bioprinting, an advanced tissue engineering technology, addresses these limitations by creating custom-designed tissue scaffolds using biomaterial-based bioinks containing living cells. This approach provides a more physiologically relevant 3D structure and microenvironment, allowing the incorporation of microbial communities to better reflect in vivo conditions. Here, we present a protocol for 3D bioprinting an in vitro skin infection model by co-culturing human keratinocytes and dermal fibroblasts in a high-viscosity, fibrin-based bioink to mimic the dermis and epidermis. The bioprinted skin tissue was co-infected with Staphylococcus aureus and Staphylococcus epidermidis to mimic bacterial skin disease. Bacterial survival was assessed through colony-forming unit enumeration. By incorporating bacteria, this protocol offers the potential to serve as a more representative in vivo 3D bioprinted skin infection model, providing a platform to study host–microbe interactions, immune responses, and the development of antimicrobial therapeutics.
Key features
• This protocol provides a detailed description of the cell culture process for both keratinocyte and fibroblast cells.
• This protocol outlines step-by-step preparation of the high-viscosity fibrin bioink and chemical crosslinker.
• The protocol uses an extrusion-based bioprinter, with an easy-to-follow methodology that clarifies the printing details, including the incorporation of skin cells into the bioink.
• This protocol details how the bacteria are inoculated into the construct to achieve the co-infection 3D skin model.
Keywords: 3D bioprinting (三维生物打印)Graphical overview
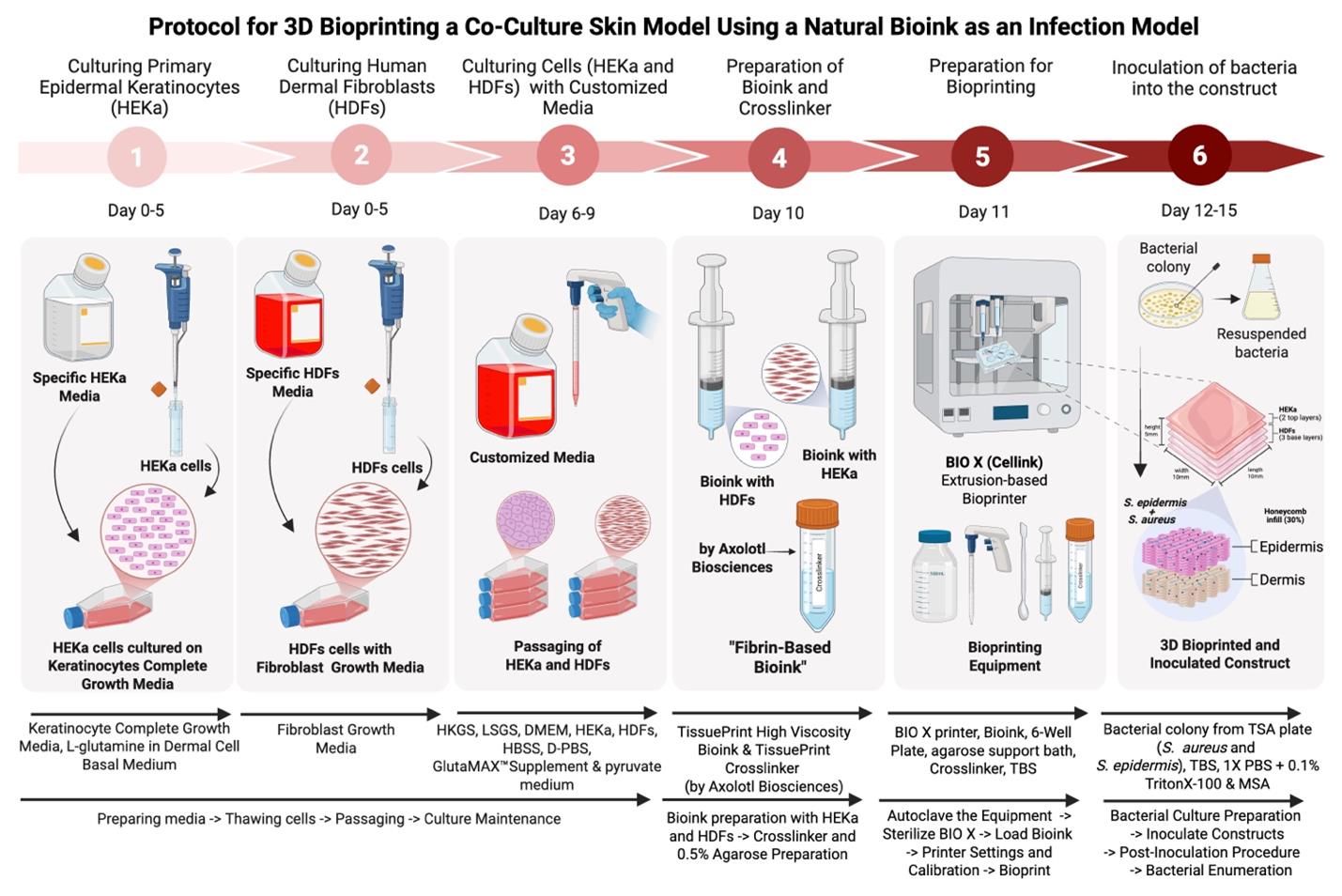
Background
The skin of humans and other mammals hosts a highly diverse microbial community that plays a critical role in maintaining skin homeostasis and defending against pathogens [1,2]. Commensal microorganisms engage in protective innate and adaptive immune mechanisms and act as a first line of defense against external stressors such as pH fluctuations and temperature changes [3,4]. For example, Staphylococcus epidermidis (S. epidermidis), a coagulase-negative staphylococci (CoNS) species, secretes extracellular serine protease (Esp), which inhibits Staphylococcus aureus (S. aureus) colonization by degrading proteins essential for adhesion and biofilm formation. Additionally, S. epidermidis stimulates keratinocytes to release antimicrobial peptides (AMPs), such as β-defensins and cathelicidin, which undermine S. aureus biofilms [5].
The skin maintains a delicate equilibrium between commensal microbes, skin cells, and immune cells, dynamically adapting to environmental changes to preserve barrier function [6]. Disruptions to this balance—caused by environmental stressors, infectious agents, or genetic mutations—can lead to dysregulation of the host-microbiome equilibrium, increasing susceptibility to skin infections and chronic inflammatory conditions such as atopic dermatitis (AD) [7,8]. Despite the critical role of the skin microbiome in health and disease, the precise mechanisms by which it maintains barrier homeostasis and defends against pathogens remain poorly understood [9].
Advanced in vitro models that accurately replicate the complex skin microenvironment and microbiome are needed to understand these interactions and competition between the skin microbiome, invading opportunistic pathogens, and organisms that do both (pathobionts) [10]. Traditional two-dimensional (2D) cell cultures and animal models have significant limitations. While 2D cultures lack physiological complexity, animal models are hindered by species-specific differences and ethical concerns [8]. Three-dimensional (3D) bioprinting has emerged as a powerful alternative, enabling the creation of in vitro skin models that more closely mimic human in vivo conditions. Using bioinks—biocompatible hydrogels containing living cells—3D bioprinting allows for precise spatial organization and the formation of tissue-like structures, including multi-layered constructs that replicate the dermis and epidermis [11,12]. These models support cell proliferation, differentiation, and physiologically accurate cell interactions, making them ideal for studying bacterial infections and host responses [13,14].
Recent studies have used 3D bioprinted skin models to investigate bacterial infections. For example, Villata et al. developed a 3D dermis–epidermis model using gelatin methacryloyl (GelMA) to study wound healing and bacterial infections [15]. However, a recent study by Rashad et al. highlighted that the high viscosity of GelMA in 3D bioprinting can negatively impact critical factors such as cell viability and morphology [16]. Our protocol employs a fibrin-based bioink, which offers superior biocompatibility and promotes better cell survival and structural integrity [17]. Similarly, Kohda et al. used a 3D epidermal model to study the growth dynamics of S. epidermidis and S. aureus, demonstrating that commensal bacteria can inhibit pathogenic growth and reduce epidermal cytotoxicity [18].
While these studies provide valuable insights, they are limited by their focus on single-layer models or the use of less physiologically relevant materials. Our protocol addresses these limitations by presenting a method for 3D bioprinting a multi-layered human skin model using a fibrin-based bioink. The model incorporates both keratinocytes and fibroblasts to replicate the dermal and epidermal layers of human skin. By co-infecting the model with S. epidermidis and S. aureus, we aim to study the interactions between these pathobionts that cohabitate the skin, in a physiologically relevant context. Bacterial survival is quantified through colony-forming unit (CFU) enumeration over a 72-h period. This approach provides a platform for investigating host–microbe interactions, immune responses, and the development of antimicrobial therapies.
The fibrin-based bioink used in our protocol, TissuePrint, has been successfully employed in 3D bioprinting of neural tissue models, demonstrating its versatility and biocompatibility [19–22]. Unlike previous protocols, ours focuses on co-culturing human skin cells to mimic the layered structure of human skin and introduces a detailed method for infecting 3D bioprinted constructs with skin bacteria. This makes our protocol particularly valuable for generating infection models and studying skin microbiome dynamics.
Despite its advantages, our model has some limitations. The lack of vascularization restricts its ability to fully replicate in vivo cellular responses and bacterial interactions. Additionally, while CFU enumeration provides insights into bacterial survival, it does not capture spatial distribution or the precise role of bacteria in skin barrier function. Future studies could incorporate assays such as lactate dehydrogenase (LDH) release to assess keratinocyte cytotoxicity and further elucidate the protective role of commensal bacteria [18]. Another limitation is the degradation of constructs during extended culture periods, which could be addressed by optimizing culture conditions to allow for epidermal maturation, as demonstrated by Liu et al. [23]. In conclusion, our protocol offers a physiologically accurate 3D bioprinted skin model that incorporates the skin microbiome, providing a valuable tool for studying host-microbe interactions, skin infections, and the development of novel therapies. While challenges remain, this model represents a significant advancement over traditional 2D cultures and animal models, with broad applications in dermatological research.
Materials and reagents
Biological materials
1. Primary epidermal keratinocytes (HEKa) (ATCC, catalog number: PCS-200-011)
2. Human dermal fibroblasts (HDFs) (Cell Applications Inc., catalog number: 106-05a)
3. Staphylococcus epidermidis strain RP62a (BEI Resources, catalog number: NR-45879) [24]
4. Staphylococcus aureus strain MW2 (BEI Resources, catalog number: NR-46054) [25]
Reagents
1. Dermal cell basal media (ATCC, catalog number: PCS 200-030)
2. Keratinocyte Growth kit (ATCC, catalog number: PCS 200-040), containing: bovine pituitary extract (BPE), 2.0 mL, 0.4%; rh TGF-a, 0.5 mL, 0.5 ng/mL; L-Glutamine, 15.0 mL, 6 mM; hydrocortisone hemisuccinate, 0.5 mL, 100 ng/mL; rh insulin, 0.5 mL, 5 μg/mL; epinephrine, 0.5 mL, 1.0 μM; Apo-Transferrin, 0.5 mL, 5 μg/mL
3. Fibroblast growth media (Cell Applications Inc., catalog number: 116-500)
4. Dulbecco’s modified Eagle medium (DMEM) high glucose, GlutaMAXTM supplement, pyruvate (Thermo Fisher Scientific, catalog number: 10569-010)
5. Human keratinocyte growth supplement (HKGS) (Thermo Fisher Scientific, catalog number: S001)
6. Low serum growth supplement (LSGS) (Thermo Fisher Scientific, catalog number: S00310)
7. Trypan blue (Thermo Fisher Scientific, catalog number: 15250-061)
8. Trypsin-ethylenediaminetetraacetic acid (EDTA) (for fibroblasts) (Cell Applications Inc., catalog number: 070-100)
9. Trypsin-ethylenediaminetetraacetic acid (EDTA) (for keratinocytes) (ATCC, catalog number: PCS 999 003)
10. Trypsin neutralizing solution (for fibroblasts) (Cell Applications Inc., catalog number: 070-100)
11. Fetal bovine serum (FBS) (R&D Systems, catalog number: S12450)
12. Dulbecco’s phosphate-buffered saline (DPBS) (R&D Systems, catalog number: B30250)
13. Hank’s balanced salt solution (HBSS) (Thermo Fisher Scientific, catalog number: 14-175-095)
14. Phosphate buffer solution (PBS) (Thermo Fisher Scientific, catalog number: 10010-023)
15. Tris hydrochloric acid (Tris HCl) (VWR Chemicals, catalog number: 22J175305)
16. Tris(hydroxymethyl)aminomethane (Tris base) (Thermo Fisher Scientific, catalog number: 198252)
17. Sodium chloride (NaCl) (Acros Organic, catalog number: A0425202)
18. Potassium chloride (KCl) (Caledon, catalog number: 73495)
19. Distilled water
20. Froggarose LE (FroggaBio, catalog number: A87-500G)
21. Triton X-100 (Sigma-Aldrich, catalog number: 9036-19-5)
22. Ethanol, 95% (Greenfield Global, catalog number: 9036-19-5)
23. TissuePrint (Axolotl Biosciences, catalog number: AX001)
24. TissuePrint Crosslinker (Axolotl Biosciences, catalog number: AX002)
25. Mannitol salt agar (BD, catalog number: BD 211407)
26. Tryptic soy agar (Millipore Sigma, catalog number: 22091)
27. Tryptic soy broth (Millipore Sigma, catalog number: 22092)
Solutions
1. Tris-buffered saline (TBS) solution (see Recipes)
2. 0.1% Triton-X (see Recipes)
3. 70% ethanol solution (see Recipes)
4. 5% trypsin neutralizing solution (for keratinocytes) (see Recipes)
Recipes
1. Tris-buffered saline (TBS) solution
To make 4 L of TBS solution, dissolve the following reagents in distilled water while stirring on a magnetic stir plate. Adjust the pH to 7.7. The prepared TBS is kept at room temperature and is disposed of after use in this protocol.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris HCl | 27.7 mM | 17.44 g |
| Tris base | 5.3 mM | 2.56 g |
| NaCl | 136.9 mM | 32 g |
| KCl | 2.7 mM | 0.8 g |
| Distilled water | n/a | 4 L |
2. 0.1% Triton-X solution
To prepare 25 mL of 0.1% Triton-X, mix the following reagents in a 50 mL conical tube and vortex until fully dissolved. Triton X-100 (100%) can be stored at room temperature for 24 months.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PBS | n/a | 24.975 mL |
| Triton X-100 (100%) | 0.1% | 25 μL |
3. 70% ethanol solution
To prepare 5.43 L of 70% ethanol solution, mix the following reagents in a large plastic container. The prepared ethanol is kept at room temperature until it is fully consumed.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ethanol (95%) | 70% | 4 L |
| Distilled water | n/a | 1.43 L |
4. 5% trypsin neutralizing solution (for keratinocytes)
To prepare 10 mL of a 5% trypsin neutralizing solution, mix the following reagents in a 50 mL conical tube inside a biological safety cabinet (BSC). The prepared solution is stored at 4 °C for up to 2 weeks.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| FBS | 5% | 500 μL |
| PBS | n/a | 9.5 mL |
Laboratory supplies
1. T-75 Corning flasks (Corning, catalog number: CLS430641U-100EA)
2. Serological pipettes 5 mL (Falcon, catalog number: 357543)
3. Serological pipettes 10 mL (Fisherbrand, catalog number: 13-678-11)
4. Serological pipettes 25 mL (Fisherbrand, catalog number: 13-678-11E)
5. 5 mL syringe (Terumo, catalog number: SS-05L)
6. 10 mL syringe (BD, catalog number: 302995)
7. 100 mL graduated cylinder
8. 250 mL glass beaker
9. Glass pipette
10. Magnetic stir plate and stir bar
11. 50 mL conicals (Fisherbrand, catalog number: 05-539-13)
12. 15 mL conicals (Falcon, catalog number: 352196)
13. Autoclavable containers
14. Reusable glass media bottles with cap (100 mL, 250 mL, 1000 mL) (Fisherbrand, catalog number: FB-800-100)
15. 0.2 μm cellulose acetate sterile syringe filter (Sartorius, catalog number: 17823----------K)
16. 0.2 μm PTFE syringe filter (VWR, catalog number: 76479-044)
17. Parafilm (Amcor, catalog number: PM-996)
18. 4 L container (VWR, catalog number: ca7350-022)
19. P1000, P200, and P20 pipettes (Axygen, catalog numbers: BT-1000-R-S, BT-250-R-S, BT-50-R-S)
20. Pipette tips (20 μL, 200 μL, and 1000 μL)
21. pH meter
22. Conical rack
23. Metal spatula
24. 500 mL beaker
25. 200 mL beaker
26. 250 mL beaker
27. 6-well cell culture plate (Cellstar, catalog number: 657160)
28. 12-well cell culture plate (Cellstar, catalog number: 662160)
29. Metal Luer locks
30. Sterile empty cartridges with end and tip caps, 3 mL (CELLINK, catalog number: CSC010311101)
31. Sterile standard blunt needles 22G (CELLINK, catalog number: NZ5220255001)
32. Kimwipes
33. Waste container
Equipment
1. BIO X printer (CELLINK, catalog number: D16110020717, fitted with a 3 mL pneumatic printhead bioprinting nozzle
2. Microscope (Leica, model: DMI3000B)
3. FormaTM Steri-CycleTM CO2 incubator (Thermo Fisher, model: 370)
4. Biosafety Cabinet (Microzone, model: BK-2-4)
5. Water bath (VWR, catalog number: 89032-299)
6. Centrifuge 5810 R (Eppendorf, catalog number: 5811000015)
7. DeNovix CellDrop FL (FroggaBio, model: DeNovix CellDrop FL)
8. Autoclave (Market Forge, model: STM-EL)
9. Cytation 5 Cell Imaging Multimode Reader (BioTek/Agilent, model: Cytation 5)
Software and datasets
1. QCapture Software (QImaging, version 2.9.12), QImaging (https://www.qimaging.com)
2. Excel (Microsoft, latest version), Microsoft (https://www.microsoft.com/en-us/microsoft-365/excel)
3. ImageJ (National Institutes of Health, version 1.52a), NIH (https://imagej.nih.gov/ij/); open source
4. Clipchamp Software (Microsoft, latest version), Microsoft (https://www.clipchamp.com)
5. Fliki AI (Fliki, latest version), Fliki (https://www.fliki.ai)
6. GraphPad Prism 10 (GraphPad Software, version 10), GraphPad Software (https://www.graphpad.com)
Procedure
文章信息
稿件历史记录
提交日期: Mar 18, 2025
接收日期: Jun 12, 2025
在线发布日期: Jul 3, 2025
出版日期: Jul 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Díaz, G. Y., Perry, M. A., Cárdenas, L. S., Da Silva, V. A., Scheck, K., Tschofen, S. A., Tuffs, S. W. and Willerth, S. M. (2025). Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model. Bio-protocol 15(14): e5380. DOI: 10.21769/BioProtoc.5380.
分类
生物工程 > 生物打印
微生物学 > 抗微生物试验 > 抗细菌试验
细胞生物学 > 细胞分离和培养 > 3D细胞培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














