- EN - English
- CN - 中文
Quantification of Neural Progenitor Cells From Zika Virus-Infected Zebrafish Embryos
寨卡病毒感染斑马鱼胚胎中神经前体细胞的定量分析
发布: 2025年07月05日第15卷第13期 DOI: 10.21769/BioProtoc.5377 浏览次数: 1882
评审: Ivonne SehringAnonymous reviewer(s)

相关实验方案

基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1658 阅读
Abstract
Zika virus (ZIKV), an arthropod-borne orthoflavivirus, has emerged as a global health concern due to its ability to cause severe fetal neurological disorders, leading to the congenital Zika syndrome (CZS) in neonates. Vertical transmission during pregnancy can alter neural progenitor cell (NPC) proliferation and differentiation and induce apoptosis, leading to microcephaly and other neurodevelopmental abnormalities. While mammalian models have been used to study the impact of ZIKV on NPC behavior, limitations such as high costs, dedicated time, and ethical constraints have fostered the exploration of alternative systems. The zebrafish embryo constitutes an advantageous in vivo model for studying ZIKV neuropathogenesis. Indeed, ZIKV infection phenocopies several features of the CZS while sharing a conserved neuroanatomical layout and offering genetic plasticity and unique accessibility to the infected brain compared to mammals. Here, we describe a protocol for characterizing ZIKV-induced defects of NPCs in this zebrafish model, relying on whole animal flow cytometry.
Key features
• This protocol introduces a novel and straightforward zebrafish-based system for studying ZIKV-induced neuropathogenesis.
• The protocol employs an unbiased methodology for flow cytometry-based quantification of neural progenitor cells from whole embryos.
• This protocol can be transposed to any transgenic reporter zebrafish lines producing specific fluorescent cell populations.
Keywords: Zebrafish (斑马鱼)Graphical overview
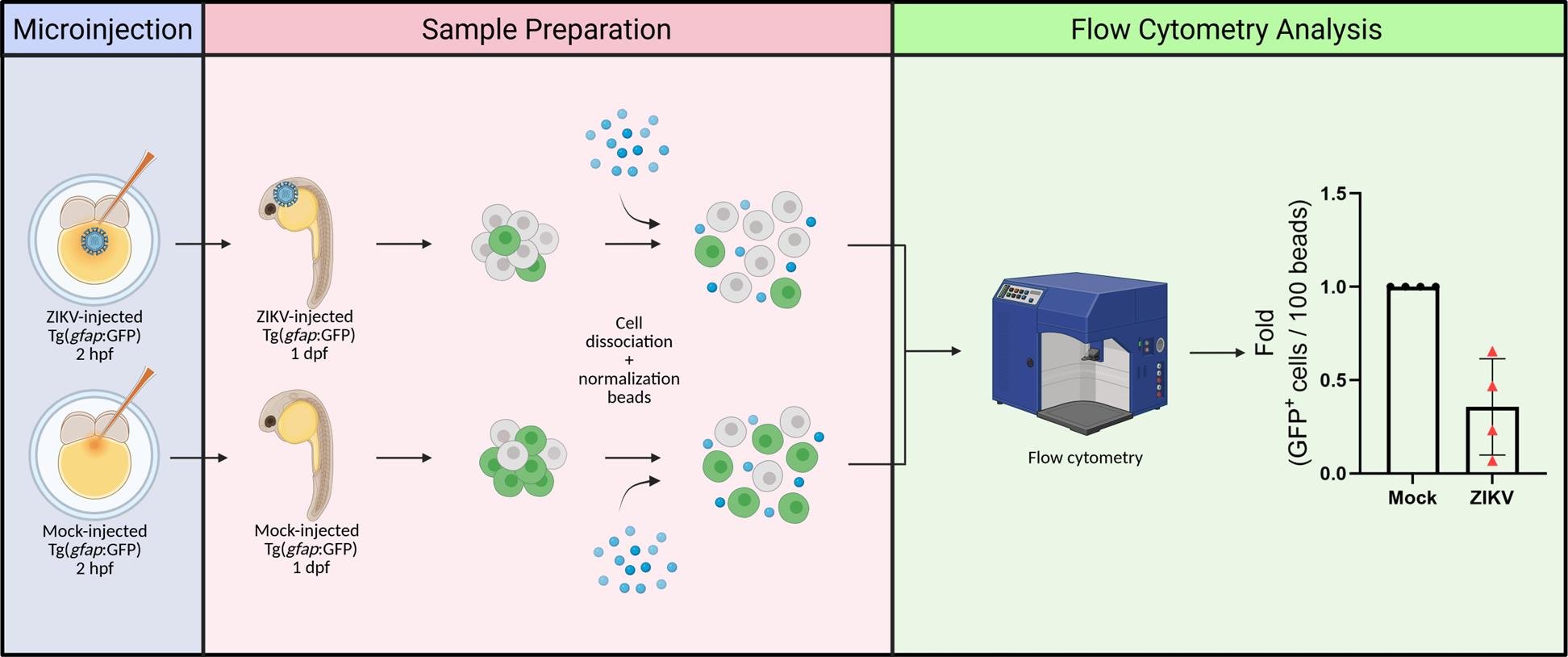
Background
Zika virus (ZIKV) is an arthropod-borne RNA virus belonging to the Orthoflavivirus genus. Public health concerns surrounding ZIKV infection arose during the massive outbreak of 2015–2016 in Brazil [1], since it was linked to severe neurological disorders in neonates—later termed congenital Zika syndrome (CZS)—that included microcephaly and were observed after vertical transmission in infected pregnant women [2,3].
Throughout gestation, ZIKV can cross the placental barrier, reach the developing fetal central nervous system, and target brain cells such as neural progenitor cells (NPCs) [4]. Studies using in vivo mouse models have notably shown that ZIKV infects NPCs, resulting in cell cycle arrest, premature differentiation, and cell death, which alter fetal neurodevelopment and thus likely contribute to microcephaly and more generally to the CZS [5,6].
Deepening into these cellular effects requires robust animal models to investigate NPC behavior. However, mammalian systems often present significant challenges due to major limitations such as costs, dedicated time, ethical considerations, difficulties in retrieving specific cell types within the brain, and limited genetic plasticity, potentially leading to embryonic lethality. On the other hand, the zebrafish has emerged as a powerful and cost-effective, easy-to-image, rapidly developing model for phenocopying and studying human neuropathological and viral diseases [7,8].
The larval zebrafish is ideally suited to examine brain neurodevelopment as it shares a basic neuroanatomical layout with that of mammals [9]. Zebrafish transgenic lines have demonstrated their utility in imaging, isolating, and characterizing specific neural populations, such as NPCs [Tg(gfap:GFP) and Tg(nestin:GFP)], neural crest cells [Tg(sox10:EGFP)], oligodendrocytes [Tg(olig2:EGFP) and Tg(mbp: EGFP)], post-mitotic neurons [Tg(elavl3:EGFP], and others [10]. By exploiting these advantages, our team has recently reported a novel zebrafish-based in vivo model of ZIKV neuropathogenesis [11]. In this system, zebrafish larvae injected with viral infectious particles exhibit developmental defects. ZIKV infection of NPCs in midbrain and forebrain cryosections correlates with a decrease in head size (i.e., microcephaly) and NPC abundance, as well as induction of apoptosis in the brain and NPC mislocalization, thus phenocopying the disease in humans and mice. NPC-focused analyses of immunolabeled brain cryosections were complemented by a fluorescence activated cell sorting (FACS)-based approach using Tg(gfap:GFP) and Tg(nestin:GFP) NPC reporter fish lines, which allows the quantification of the abundance of NPCs as well as their isolation from whole embryos for subsequent analysis such as bulk or single-cell mRNA sequencing [11]. Here, we provide a detailed description of the procedures for this unbiased NPC quantification in ZIKV-infected and control Tg(gfap:GFP) zebrafish embryos at one day post-fertilization.
Materials and reagents
Biological materials
1. Zebrafish (Danio rerio) lines: Tg(gfap:GFP) [12], expressing GFP under the gfap promoter; and parental wildtype TL
Note: Although this protocol utilizes only the Tg(gfap: GFP) line, it can also be applied, resulting in similar findings, with Tg(nestin: GFP) transgenic embryos, which express GFP under the nestin promoter and also exhibit fluorescent NPCs.
2. Zika virus (ZIKV) strain H/PF/2013 (European Virus Archive Global, catalog number: 001v-EVA1545) (stocks produced and titered in VeroE6 cells according to the procedure described in our previous Bio-protocol article by Freppel et al. [13]).
Reagents
1. Dulbecco’s modified Eagle medium (DMEM) (Fisher Scientific, catalog number: 11965118)
2. Fetal bovine serum (FBS) performance (Wisent, catalog number: 098150)
3. Penicillin-streptomycin (Life Technologies, catalog number: 15140-122)
4. MEM non-essential amino acids solution (100×) (Life Technologies, catalog number: 11140-050)
5. Milli-Q water
6. Phosphate-buffered saline (PBS) (Fisher Scientific, catalog number: AAJ67653AP, stored at 4 °C)
7. Mineral oil (MilliporeSigma, catalog number: M8410)
8. CaCl2 (MilliporeSigma, catalog number: C4901)
9. MgCl2 (MilliporeSigma, catalog number: 208337)
10. NaCl (Fischer Scientific, catalog number: S271-3)
11. KCl (Fischer Scientific, catalog number: BP366-500)
12. NaHCO3 (MilliporeSigma, catalog number: S5761)
13. NaOH (Fischer Scientific, catalog number: S318-1)
14. FACSmax cell dissociation solution (AMSBIO, catalog number: T200100, aliquoted and stored at -20 °C)
15. 123count eBeads counting beads (Fisher Scientific, catalog number: 501129030, stored at 4 °C in the dark)
16. Formaldehyde (FA) solution 37% (MilliporeSigma, catalog number: F8775)
Solutions
1. 60× E3 medium (see Recipes)
2. Deyolking buffer (see Recipes)
3. 4% FA/PBS (see Recipes)
4. Complete DMEM (see Recipes)
Recipes
1. 60× E3 medium
Prepare 2 L of E3 stock solution 60× (pH 7.2), autoclave, and store at 4 °C for a maximum period of 6 months. Then, prepare 1× E3 medium by diluting the stock solution in Milli-Q water and store at room temperature.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MgCl2 | 51.36 mM | 9.78 g |
| CaCl2 | 26.13 mM | 5.8 g |
| NaCl | 297.74 mM | 34.8 g |
| KCl | 10.73 mM | 1.6 g |
| Milli-Q water | n/a | Up to 2 L |
| Total | n/a | 2 L |
2. Deyolking buffer
Prepare 100 mL of deyolking buffer and store at 4 °C for a maximum period of 6 months.
| Reagent | Final concentration | Volume |
|---|---|---|
| NaCl 4 M | 55 mM | 1,375 µL |
| KCl 1 M | 1.8 mM | 180 µL |
| NaHCO3 0.5 M | 1.25 mM | 250 µL |
| Milli-Q water | n/a | Up to 100 mL |
| Total | n/a | 100 mL |
3. 4% FA/PBS
Prepare 100 mL of 4% FA/PBS solution, aliquot, and store at -20 °C until use for a maximum period of 2 years.
| Reagent | Final concentration | Volume |
|---|---|---|
| Formaldehyde 37% | 4% | 10.8 mL |
| PBS | n/a | Up to 100 mL |
| Total | n/a | 100 mL |
4. Complete DMEM
Prepare 500 mL of complete DMEM, aliquot, and store at -80 °C for a maximum period of 3 years (although storage time is not expected to alter this solution).
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | n/a | 440 mL |
| FBS | 10% | 50 mL |
| Penicillin-streptomycin | 100 U/mL–100 mg/mL (1%) | 5 mL |
| MEM non-essential amino acids | 1% | 5 mL |
| Total | n/a | 500 mL |
Laboratory supplies
1. Plastic transfer pipettes (Fisher Scientific, catalog number: 137117MMD)
2. 2–20 µL pipette (Avantor, catalog number: 613-5260)
3. Pipette tips (Vertex, catalog number: 4117NSF)
4. Borosilicate glass, 1.0 mm × 0.5 mm × 10 cm, with filament (Sutter Instrument, catalog number: BF100-50-10)
5. Microloader tips (Eppendorf, catalog number: 930001007)
6. 0.01 mm microscope stage calibration slide (AmScope, catalog number: MR095)
7. Glass microscopy slides (Ultident, catalog number: 170-8105-W+)
8. 10 cm Petri dishes (Falcon, catalog number: 353003)
9. 6-well plates (Corning, catalog number: 3506)
10. Cell strainers (Falcon, catalog number: C352350)
11. Syringes (BD, catalog number: 309628)
12. Falcon tubes (Falcon, catalog number: 352098)
13. Forceps (Fine Science Tools, catalog number: 11252-00)
14. Flow cytometry tubes (Falcon, catalog number: 352008)
Equipment
1. Breeding tank (Tecniplast, catalog numbers: ZB10BTE, ZB10BTI, ZB10BTD, ZB10BTL)
2. Micropipette puller (Sutter Instrument, model: P-97)
3. Stereomicroscope (Zeiss, model: Stemi 305)
4. Microinjector (Eppendorf, model: FemtoJet 4i)
5. Incubator (Boekel Scientific, model: 131400)
6. Vortex mixer (Fisherbrand, model: Analog Vortex Mixer)
7. Centrifuge (Thermo Scientific, model: Sorvall ST 16R)
8. Flow cytometer (BD, model: LSRFortessa Cell Analyzer, model: 647794L6)
Software and datasets
1. FlowJo version 10.8.1 (BD Life Sciences)
2. GraphPad Prism version 10.0.0 (Dotmatics)
Procedure
文章信息
稿件历史记录
提交日期: Apr 20, 2025
接收日期: Jun 6, 2025
在线发布日期: Jun 19, 2025
出版日期: Jul 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Gomes, Y. C. P., Sow, A. A., Patten, S. A. and Chatel-Chaix, L. (2025). Quantification of Neural Progenitor Cells From Zika Virus-Infected Zebrafish Embryos. Bio-protocol 15(13): e5377. DOI: 10.21769/BioProtoc.5377.
分类
神经科学 > 神经系统疾病 > 动物模型
细胞生物学 > 基于细胞的分析方法 > 病毒性感染
神经科学 > 发育 > 形态建成
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











