- EN - English
- CN - 中文
Rapid and Multiplex Diagnosis of Malaria Using Chelex-100 Extraction and LAMP-MS Assay
基于Chelex-100提取与LAMP-MS检测的疟疾快速多重诊断方法
发布: 2025年07月05日第15卷第13期 DOI: 10.21769/BioProtoc.5375 浏览次数: 1816
评审: Ivonne SehringManousos E. KambourisAnonymous reviewer(s)
Abstract
Malaria remains a major public health threat, especially in tropical and subtropical regions. Accurate and rapid diagnosis is essential for effective disease management and control, yet conventional malaria diagnostics, including blood smear microscopy using Giemsa staining, PCR, and rapid diagnostic tests (RDTs), are limited by the need for trained personnel, reliance on laboratory infrastructure, and reduced sensitivity at low parasite densities, respectively. This protocol details an innovative, rapid, and economical diagnostic platform combining a simplified Chelex-100 resin-based nucleic acid extraction method with a multiplex loop-mediated isothermal amplification microscanner (LAMP-MS) assay. The malaria diagnostic platform enables simultaneous detection of Plasmodium falciparum (Pf), Plasmodium vivax (Pv), pan-malaria (Pan), and an internal control (IC) within 40 min, from DNA extraction to result interpretation. It demonstrates sensitivity and specificity comparable to traditional PCR-based diagnostics, making it a practical and scalable solution for use in resource-constrained environments.
Key features
• Low-cost and simple DNA extraction using Chelex-100 resin.
• Multiplex detection of Pan malaria, Pf, Pv, and IC using microchip chambers.
• Visual endpoint detection using a microscope or a microscanner.
• Field-deployable with minimal equipment.
Keywords: Malaria (疟疾)Graphical overview
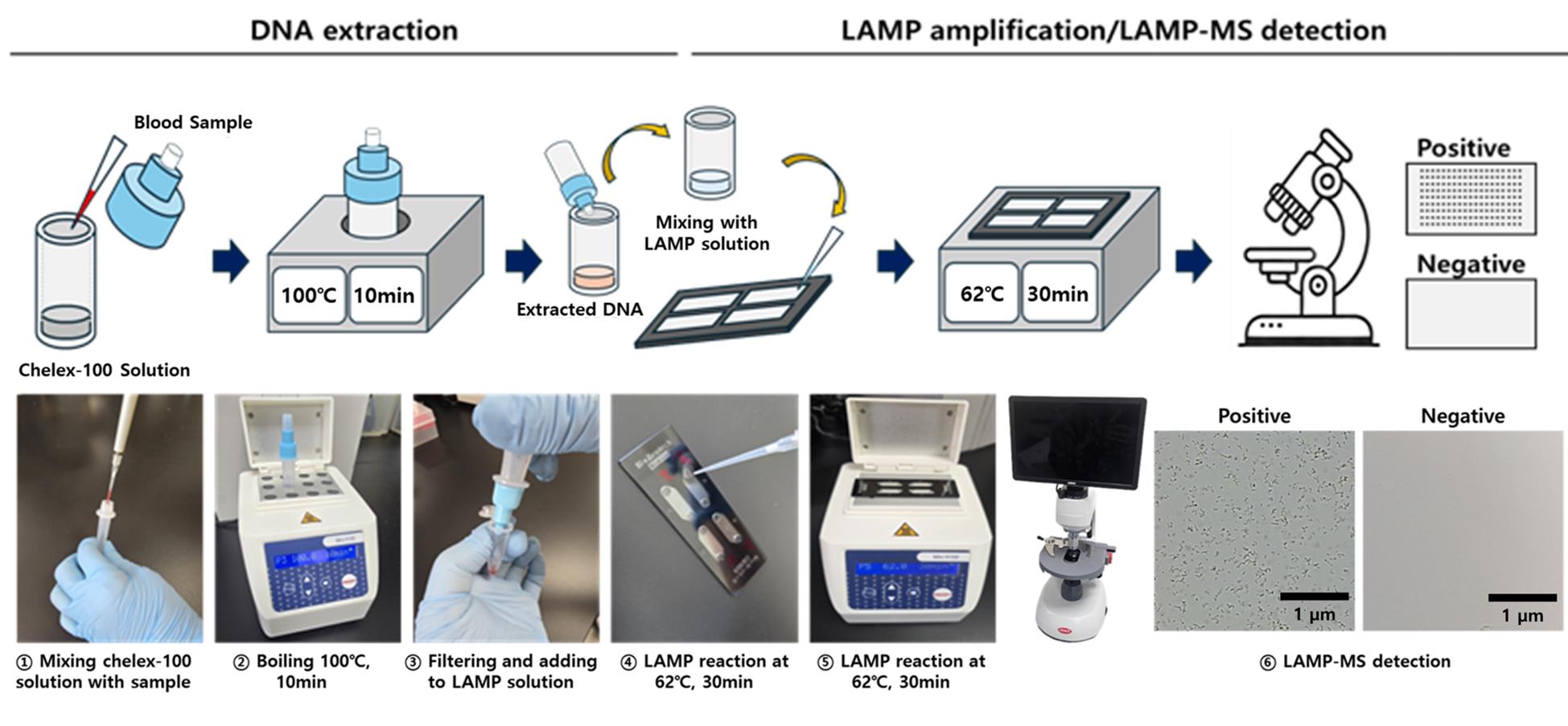
Schematic illustration of the malaria Pan/Pf/Pv/IC loop-mediated isothermal amplification microscanner (LAMP-MS) assay
Background
Malaria continues to be a major global public health challenge, particularly in tropical and subtropical regions [1]. In 2020 alone, an estimated 241 million cases and over 627,000 deaths were reported, with the majority of fatalities occurring among children under five in sub-Saharan Africa [2,3]. Early and accurate diagnosis is critical not only for initiating timely treatment but also for controlling disease transmission and reducing associated mortality and morbidity [4].
Conventional malaria diagnostic methods, such as blood smear microscopy using Giemsa staining, rapid diagnostic tests (RDTs), and polymerase chain reaction (PCR), each present distinct strengths and limitations. Blood smear microscopy is cost-effective and widely accessible but requires trained personnel and is subject to inter-operator variability [5]. PCR offers high sensitivity and specificity but depends on advanced laboratory infrastructure and skilled technicians, making it impractical in many low-resource settings [6,7]. RDTs, though widely adopted for their simplicity and speed, may exhibit reduced sensitivity in cases of low parasite density [8]. To overcome these limitations, loop-mediated isothermal amplification (LAMP) has emerged as a promising alternative. LAMP employs three pairs of primers: inner primers (FIP/BIP), outer primers (F3/B3), and loop primers (LoopF/LoopB). The inner and outer primers initiate strand displacement amplification, while the loop primers enhance the reaction speed by promoting faster cycling. LAMP offers sensitivity and specificity comparable to PCR while requiring only minimal equipment, making it highly suitable for field-based applications in endemic areas [9–11].
Building on this potential, our research group has developed a novel diagnostic platform that integrates a Chelex-100/boiling-based DNA extraction method with a microchip-based LAMP-MS (microscanner) assay [12]. As illustrated in the Graphical overview, the LAMP-MS workflow begins with heat-based DNA extraction using Chelex-100, followed by multiplexed LAMP reaction in a microchip, and visual endpoint detection under a microscope. This system allows for rapid, multiplex detection of Plasmodium falciparum, P. vivax, and pan-malarial targets through visually interpretable results without the need for complex instrumentation.
This protocol presents a practical and scalable approach that combines Chelex-100 resin-based nucleic acid extraction with a microchip-integrated LAMP-MS assay, enabling simultaneous detection of multiple malaria species. Its simplicity, cost-effectiveness, and rapid visual readout make this platform especially well-suited for resource-limited settings [12].
Materials and reagents
Biological materials
Malaria residual blood samples were collected from patients suspected of malaria infection at Korea University Guro Hospital between 2013 and 2022. Whole blood samples were collected from patients using standard venipuncture techniques into EDTA-containing tubes, gently mixed, and stored at 4 °C until further processing. After routine diagnostic testing, they were stored at -80 °C. Malaria infection was confirmed through various methods, including Giemsa staining, blood smear microscopy, rapid antigen tests, and PCR tests. Procedures involving clinical samples, including handling and nucleic acid extraction, were conducted under Biosafety Level 2 (BSL-2) conditions. This study was approved by the Medical Ethics Committee of Korea University’s Guro Hospital (2021GR0337).
1. 80 P. falciparum clinical samples
2. 80 P. vivax clinical samples
3. 100 negative clinical samples
4. Zika virus, Chikungunya virus, and Japanese encephalitis virus, provided by the Korea Disease Control and Prevention Agency (KDCA)
Reagents
1. Chelex-100 resin (Bio-Rad, catalog number: 142-1253)
2. Tris-EDTA buffer solution (Sigma-Aldrich, catalog number: T9285)
3. Phosphate-buffered saline (PBS) (Thermo Fisher Scientific, catalog number: 10010-023)
Solutions
1. LAMP primer mix (see Recipes)
Recipes
1. LAMP primer mix
a. Prepare primer solutions separately for each target (Pan, Pf, Pv, IC) at the following final concentrations:
i. 4 μM of each outer primer (F3 and B3)
ii. 32 μM of each inner primer (FIP and BIP)
iii. 10 μM of each loop primer (FLP and BLP)
b. Each primer set solution (Pan, Pf, Pv, IC) is prepared from 100 μM stock solutions by mixing 4 μL each of F3 and B3, 32 μL each of FIP and BIP, and 10 μL each of FLP and BLP, followed by the addition of 18 μL of nuclease-free water (Table 1).
Table 1. Multi-malaria pan/Pf/Pv/IC LAMP-MS primer sets used in this study
| Target | Name | Sequence (5′-3′) | Length (mer) |
| Malaria pan (18S rRNA gene) | Malaria pan F3 | GTA TCA ATC GAG TTT CTG ACC | 21 |
| Malaria pan B3 | CTT GTC ACT ACC TCT CTT CT | 20 | |
| Malaria pan FIP | TCG AAC TCT AAT TCC CCG TTA CCT ATC AGC TTT TGA TGT TAG GGT | 45 | |
| Malaria pan BIP | CGG AGA GGG AGC CTG AGA AAT AGA ATT GGG TAA TTT ACG CG | 41 | |
| Malaria pan FLP | CGT CAT AGC CAT GTT AGG CC | 20 | |
| Malaria pan BLP | AGC TAC CAC ATC TAA GGA AGG CAG | 24 | |
| Plasmodium falciparum (LDH gene) | P. falciparum F3 | AAT TGT AAA CTT ACA TGC ATC AC | 23 |
| P. falciparum B3 | TCA AAT TTA GCT TTT TCC TCA CT | 23 | |
| P. falciparum FIP | GCC CTT CTA ACA AGG TTG AGC AGC TGC TGC TAT TAT CGA AAT | 42 | |
| P. falciparum BIP | TAT GGA CAC TCC GAT ATA TTC GGT GTA ATT CGA TAA CTT GTT CAA CAC | 48 | |
| P. falciparum FLP | CAA ATC TTT AAG TAG GAT TCA GCC | 24 | |
| P. falciparum BLP | GGT ACA CCT GTT GTT TTA GGT GCT | 24 | |
| Plasmodium vivax (16S rRNA gene) | P. vivax F3 | GGA ATG ATG GGA ATT TAA AAC CT | 23 |
| P. vivax B3 | ACG AAG TAT CAG TTA TGT GGA T | 22 | |
| P. vivax FIP | CTA TTG GAG CTG GAA TTA CCG CTC CCA AAA CTC AAT TGG AGG | 42 | |
| P. vivax BIP | AAT TGT TGC AGT TAA AAC GCT CGT AAG CTA GAA GCG TTG CT | 41 | |
| P. vivax FLP | GCT GCT GGC ACC AGA CTT | 18 | |
| P. vivax BLP | AGT TGA ATT TCA AAG AAT CG | 20 | |
| Human (actin gene) | IC F3 | AGT ACC CCA TCG AGC ACG | 18 |
| IC B3 | AGC CTG GAT AGC AAC GTA CA | 20 | |
| IC FIP | GAG CCA CAC GCA GCT CAT TGT ATC ACC AAC TGG GAC GAC A | 40 | |
| IC BIP | CTG AAC CCC AAG GCC AAC CGG CTG GGG TGT TGA AGG TC | 38 | |
| IC FLP | TGT GGT GCC AGA TTT TCT CCA | 21 | |
| IC BLP | CGA GAA GAT GAC CCA GAT CAT GT | 23 |
Laboratory supplies
1. 1.5 mL microcentrifuge tubes (Axygen, catalog number: MCT-150-C)
2. Mmiso® DNA Amplification kit master mix (Mmonitor, catalog number: 22202)
3. DNA extraction tube set (Biozentech, catalog number: NAE-03)
4. 4-chambers grid microchip (Biozentech, catalog number: BZC-04G)
5. Sealing tape (Bio-Rad, catalog number: MSB1001)
6. Aluminum foil (Taeshin Bioscience, catalog number: TS-TF3050)
Equipment
1. Micropipettes 0.1–2.5 μL, 2–20 μL, 20–200 μL (Eppendorf, catalog numbers: 3123000012, 3123000098, 3123000039)
2. Aptical RV400TMCL microscope (OMAX OMB microscope, Gyeonggi-do, Korea; field-deployable with built-in battery and monitor)
3. Microscanner (OMAX, model: RV400TMCL)
4. Vortex-Genie® 2 (Scientific Industries, catalog number: SI-0246A)
5. Heat block (BioFree, catalog number: BF-40HB)
6. Complete blood count (CBC) analyzer (Beckman Coulter, catalog number: B23858)
7. Incubator (L&T Co., catalog number: LT-201S)
8. Refrigerator (LG Electronics, catalog number: LG B607W)
Software and datasets
1. Diagnostic test calculator: https://www.medcalc.org/calc/diagnostic_test.php
Procedure
文章信息
稿件历史记录
提交日期: Apr 17, 2025
接收日期: Jun 5, 2025
在线发布日期: Jun 19, 2025
出版日期: Jul 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Lim, M. S., Choe, Y. L. and Jang, W. S. (2025). Rapid and Multiplex Diagnosis of Malaria Using Chelex-100 Extraction and LAMP-MS Assay. Bio-protocol 15(13): e5375. DOI: 10.21769/BioProtoc.5375.
分类
微生物学 > 病原体检测 > RT-LAMP
分子生物学 > DNA > PCR
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link