- EN - English
- CN - 中文
Isolation of Podocyte Cell Fractions From Mouse Kidney Using Magnetic Activated Cell Sorting (MACS)
基于磁激活细胞分选(MACS)的小鼠肾足细胞分离方法
(§Technical contact: scoobie@uw.edu) 发布: 2025年07月05日第15卷第13期 DOI: 10.21769/BioProtoc.5364 浏览次数: 2414
评审: Komuraiah MyakalaAnonymous reviewer(s)
Abstract
Glomerular diseases characterized by injury to post-mitotic epithelial cells called podocytes are a leading cause of chronic kidney disease. Yet, isolating podocytes from the kidney for transcriptomic, proteomic, and metabolomic studies has been a major technical challenge. Protocols utilizing glomerular sieving and laser capture methods are of limited use because they are not podocyte-specific but instead capture all four glomerular cell types. Here, we present a magnetic-activated cell sorting (MACS) method where podocytes are isolated from digested whole kidneys using antibodies specific to extracellular antigens on podocytes. Using microbeaded secondary antibodies binding to the podocyte-specific primary antibodies allows sorting of the podocytes using a magnet. This podocyte-only cell fraction is a unique source of in vivo–derived cells for molecular and cellular experiments.
Key features
• The protocol isolates a podocyte-only cell population from kidneys that is readily available for molecular and cellular studies.
• The non-podocyte fraction serves as a matching negative control.
• High cell yields are obtained.
• The method can be applied to separately isolate podocytes from the outer cortex and juxtamedullary regions of the kidney.
Keywords: Kidney (肾脏)Graphical overview
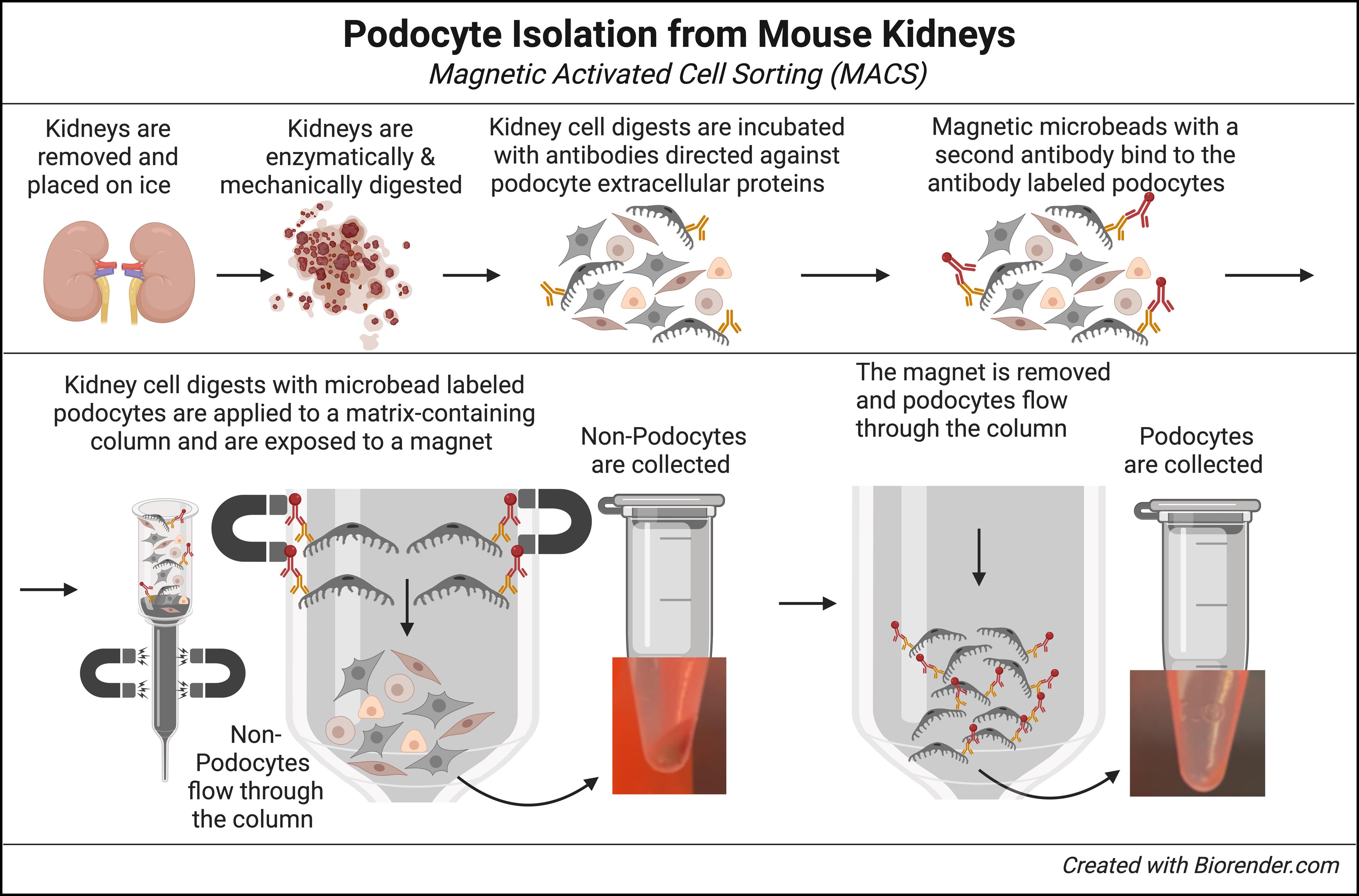
Mouse kidneys are enzymatically and mechanically digested into a single cell suspension. Surface antigens on podocytes are then labeled with a pool of specific antibodies that only bind to extracellular proteins expressed on podocytes. Magnetic microbead-conjugated anti-rabbit IgG antibodies bind to the podocyte-specific antibodies. Kidney cell suspensions containing microbead-conjugated podocytes are added to a column containing ferromagnetic spheres. Placement under a magnetic field retains podocytes in the column matrix, while unbound non-podocytes flow through and are collected. Finally, upon removal of the magnet, podocytes are flushed from the column and collected.
Background
Thanks to advances in biological technology and computing, the role of bioinformatics in biomedical research has exploded in recent years [1]. In nephrology, this has resulted in the collection of large datasets that include genome, transcriptome, proteome, and metabolome data from kidney patients, as well as from mice with experimental models of kidney disease [2–10].
However, a major challenge in collecting these data is the structural and cellular complexity of the kidney. The mouse kidney has been reported to contain >19 different cell types [11,12], and recent single-cell studies of the human kidney identified even up to 41 different cell types [11–13]. This makes it very difficult to collect data from any one specific kidney cell type. Podocytes are particularly very challenging, as these post-mitotic glomerular epithelial cells are tightly attached to the glomerular basement membrane and wrapped around the underlying vasculature.
One solution to this dilemma is the development of large-scale collections using single-cell and single-nuclear technology, which allows the extraction and analysis of individual cell types [14,15]. This methodology has some great advantages, such as the discovery of transcriptional similarities and differences within a given cell population of, e.g., healthy and diseased states, or the identification of rare cell populations that would otherwise go undetected in large pools of cells. Yet, isolating podocytes by single-cell/nuclear methods often produces low yields, and each single cell contains significantly less mRNA compared to a bulk sample of pooled cells. Thus, the detection of low-level expressed genes can be overlooked [14]. Microdissection or isolation of glomeruli and tubules can increase the granularity and depth in the data [16,17], but this approach then lacks cell type specificity.
In mice, one alternative to isolate specific kidney cell types is the use of cell-specific fluorescence reporter lines used for lineage tracing [18,19]. Performing fluorescence-activated cell sorting (FACS) on digested kidneys can obtain large pools of specific kidney cell types. Yet, limitations include the effects of long-term expression of exogenous reporters in these cells and, in the case of inducible reporters, toxic effects from the compounds used to induce expression, like tamoxifen [20], as well as the known toxicity of Cre recombinase [21–23]. Finally, a major limitation is that the vast majority of mutant and transgenic mice, and all human kidneys, lack fluorescence cell reporters.
In the protocol described herein, we provide a method for the isolation of large pools of mouse glomerular podocytes for use in bulk RNA sequencing as well as other bioinformatic applications that bypasses many of these issues.
Materials and reagents
Reagents
1. LiberaseTM TL research grade, low thermolysin (Sigma-Aldrich, catalog number: 5401020001, store at -20 °C)
2. DNase I recombinant, RNase-free (Sigma, catalog number: 4716728001, store at -20 °C)
3. Anti-nephrin, rabbit monoclonal antibody (G17-H); recognizes the epitope located between the Cys53-Cys101 extracellular domain (MyBiosource.com, catalog number: MBS684143, store at -80 °C)
4. Anti-nephrin, rabbit monoclonal antibody (Y17-R); recognizes the epitope located between the Cys465-Cys528 extracellular domain (MyBiosource.com, catalog number: MBS684100, store at -80 °C)
5. Anti-podoplanin (PDPN), rabbit monoclonal antibody; membranous mucin-type O-glycosylated glycoprotein, expressed on the surface of podocytes (MyBiosource.com, catalog number: MBS179563, store at -80 °C)
6. Anti-Rabbit IgG MicroBeads (Miltenyi Biotec, catalog number: 130-048-602, store at 4 °C)
7. Alexa Fluor® 594 AffiniPureTM donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., catalog number: 711-585-152, store at 4 °C)
8. Dulbecco’s phosphate-buffered saline, 1× with calcium and magnesium (Corning, catalog number: 21-030-CM, store at 4 °C)
9. EDTA solution, 0.5 M pH 8.0 (Invitrogen, catalog number: AM9260G, store at room temperature)
10. DEPC-treated, DNase- and RNase-free, mol. biol. water (for RNA work) (Fisher Scientific, catalog number: BP5611, store at room temperature)
11. Bovine serum albumin (BSA), lyophilized powder (Sigma-Aldrich, catalog number: A2058, store at 4 °C)
12. HyCloneTM RPMI 1640 media, liquid (Cytiva, catalog number: SH30605.01, store at 4 °C)
13. HyCloneTM 100 mM sodium pyruvate solution (Cytiva, catalog number: SH3023901, store at 4 °C)
14. Corning® Nu-SerumTM IV growth medium supplement (optional); can be replaced with standard fetal bovine serum (Corning, catalog number: 355504, store at -20 °C)
15. Penicillin-streptomycin (10,000 U/mL) (optional); increases shelf life of the podocyte wash media (Thermo Fisher, catalog number: 15140122, store at 4 °C)
16. ProLongTM Gold antifade mountant (Thermo Fisher, catalog number: P10144, store at room temperature)
Solutions
1. Podocyte wash media (see Recipes)
2. MACS buffer (see Recipes)
3. Liberase stock solution (see Recipes)
4. DNase stock solution (see Recipes)
5. Digestion buffer (Liberase/DNase working solution) (see Recipes)
Recipes
1. Podocyte wash media
Filter sterilize. Store at 4 °C and use within 2 months.
| Reagent | Final concentration | Volume |
|---|---|---|
| RPMI 1640 | Classic liquid medium | 500 mL |
| Sodium pyruvate | 1 mM | 5 mL |
| Nu-SerumTM IV growth medium supplement | 9% | 50 mL |
| Penicillin-streptomycin (optional) | 100 U/mL | 5 mL |
| Total | 560 mL |
2. MACS buffer
Make fresh. Store at 4 °C and use within 1 week.
| Reagent | Final concentration | Quantity |
|---|---|---|
| Dulbecco’s phosphate-buffered saline | 1× | 50 mL |
| EDTA solution (0.5 M) | 2 mM | 0.2 mL |
| BSA | 0.5% | 0.25 g |
| Total | 50 mL |
3. Liberase stock solution
Freeze at -20 °C, avoid repeated freezing and thawing, and use within 1 year. 0.1 mL aliquots can be used to digest two mouse kidneys.
| Reagent | Final concentration | Quantity |
|---|---|---|
| LiberaseTM TL | 10 mg/mL | 5 mg/vial |
| Water (for RNA work) | 0.5 mL |
4. DNase stock solution
Freeze at -20 °C, avoid repeated freezing and thawing, and use within 1 year. 0.05 mL aliquots can be used to digest two mouse kidneys.
| Reagent | Final concentration | Quantity |
|---|---|---|
| DNase | 10 U/mL | 10,000 U/vial |
| Total |
5. Digestion buffer (Liberase/DNase working solution)
Make fresh on the day of digestion. Keep on ice. Can be used to digest two mouse kidneys.
| Reagent | Final concentration | Volume |
|---|---|---|
| Liberase stock solution (Recipe 3) | 0.2 mg/mL | 0.1 mL |
| DNase stock solution (Recipe 4) | 100 U/mL | 0.05 mL |
| RPMI 1640 | n/a | 5 mL |
| Total | 5 mL |
Laboratory supplies
1. FisherbrandTM 10 cm Petri dishes (Fisher Scientific, catalog number: FB0875713)
2. 15 mL conical tubes (FalconTM, catalog number: 352095)
3. 30 mL conical tubes (VWR, catalog number: 76521-346)
4. 50 mL conical tubes (FalconTM, catalog number: 352070)
5. 1.7 mL microfuge tubes (VWR, catalog number: 87003-294)
6. 10 × 75 test tubes (FalconTM, catalog number: 352054)
7. FisherbrandTM sterile 10cc syringes (Fisher Scientific, catalog number: 14-955-459)
8. BD PrecisionGlideTM needle 18 G × 1-1/2 in. (Becton Dickenson, catalog number: 305196)
9. Cell strainers, 100 μm (Sigma-Aldrich, catalog number: CLS431752)
10. Cell strainers, 40 μm (Sigma-Aldrich, catalog number: CLS431750)
11. LS columns (Miltenyi Biotec, catalog number: 130-042-401)
Equipment
1. Shaking water bath (e.g., Thermo Scientific, model: 2876)
2. Roto Shake Genie or tube rotator (e.g., Scientific Industries, model: SI-1100)
3. Centrifuge (Eppendorf, model: 5424)
4. MidiMACSTM separator (Miltenyi Biotec, catalog number: 130-042-302)
5. MACS® MultiStand (Miltenyi Biotec, catalog number: 130-042-303)
Procedure
文章信息
稿件历史记录
提交日期: Mar 27, 2025
接收日期: May 18, 2025
在线发布日期: Jun 19, 2025
出版日期: Jul 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Pippin, J. W., Loretz, C. J., Eng, D. G., Wessely, O. and Shankland, S. J. (2025). Isolation of Podocyte Cell Fractions From Mouse Kidney Using Magnetic Activated Cell Sorting (MACS). Bio-protocol 15(13): e5364. DOI: 10.21769/BioProtoc.5364.
分类
细胞生物学 > 细胞分离和培养 > 细胞分离
系统生物学 > 转录组学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













