- EN - English
- CN - 中文
Primary Mouse Choroidal Endothelial Cell Culture
小鼠脉络膜内皮细胞的原代培养方法
(*contributed equally to this work) 发布: 2025年06月20日第15卷第12期 DOI: 10.21769/BioProtoc.5355 浏览次数: 2162
评审: Pilar Villacampa AlcubierreKarthik Amudhala HemanthakumarKrishna Murthy NakuluriAnonymous reviewer(s)
Abstract
The study of choroidal endothelial cells is essential for understanding the pathological mechanisms underlying choroidal neovascularization and other vision-threatening disorders. Traditional methods for isolating and culturing primary endothelial cells often yield mixed populations or require specialized equipment, limiting their widespread use. Here, we present a straightforward protocol for isolating and culturing primary mouse choroidal endothelial cells. This protocol involves enzymatic digestion of choroidal tissue, magnetic-activated cell sorting (MACS) to enrich CD31+ endothelial cells, and optimized culture conditions to promote cell proliferation and maintain endothelial phenotype. The protocol is strategic, reproducible, and requires minimal specialized equipment, making it accessible for researchers across various fields. By providing a robust method for obtaining pure choroidal endothelial cell cultures, this protocol facilitates the study of cell-specific behaviors and responses, advancing research into choroidal vascular diseases.
Key features
• Describes a protocol for isolating mouse choroidal endothelial cells (mCECs) using MatrigelTM-based explant culture followed by CD31+ cell enrichment.
• Utilizes enzymatic dissociation with dispase and filtration to achieve a single-cell suspension, ensuring high cell yield and purity.
• Confirms endothelial cell identity, enabling reliable downstream applications.
• Supports experimental induction of endothelial-to-mesenchymal transition (EndMT) using mouse transforming growth factor β2 (TGFβ2), making it suitable for studying vascular remodeling processes.
Keywords: Mouse choroidal endothelial cells (小鼠脉络膜内皮细胞)Graphical overview
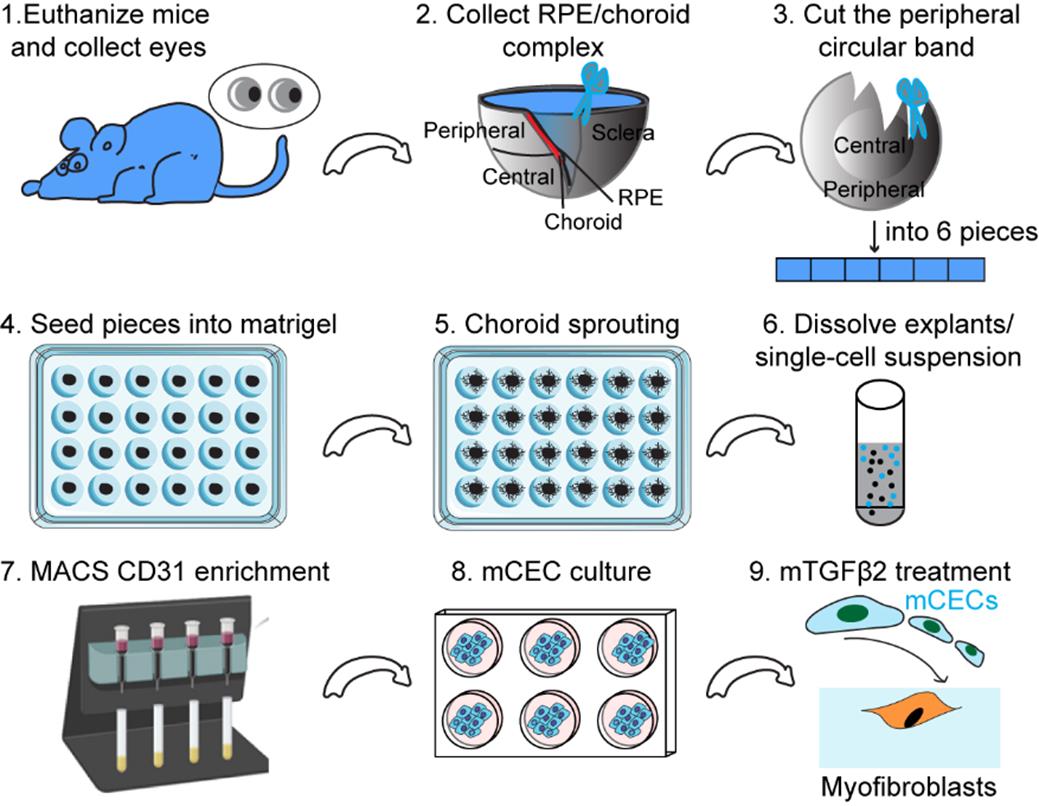
Schematic illustration of mouse choroidal endothelial cell generation and culture. This schematic illustration outlines a step-by-step process for isolating and culturing mouse choroidal endothelial cells (mCECs) from the retinal pigment epithelium (RPE)/choroid complex. First, mouse eyes are collected and dissected to isolate the RPE/choroid complex, from which the peripheral choroidal band is cut into small pieces. These tissue pieces are embedded in Matrigel to promote choroidal sprouting, and the resulting explants are enzymatically dissociated into a single-cell suspension. CD31 MicroBeads are used to isolate endothelial cells, which are then cultured in appropriate conditions to expand mCECs. Finally, mCECs are treated with mTGFβ2 to induce their transition into myofibroblasts for further study.
Background
Choroidal endothelial cells (CECs) are critical for maintaining the vascular integrity of the choroid, which supplies oxygen and nutrients to the outer retina [1,2]. Dysregulation of these cells contributes to the pathogenesis of several ocular diseases, including age-related macular degeneration (AMD) [3], diabetic retinopathy (DR) [4], oxygen-induced retinopathy (OIR) [5], and retinitis pigmentosa (PR) [6], all of which are leading causes of vision loss worldwide. Investigating the molecular mechanisms governing CEC behavior, such as angiogenesis, endothelial-to-mesenchymal transition (EndMT), and interactions with the retinal pigment epithelium, is crucial for developing targeted therapeutic strategies [7]. Thus, establishing a reliable and reproducible method for culturing CECs is critical for studying their role in disease progression.
Researchers began using ex vivo mouse choroidal tissue from isolated choroids in the early 2000s to better understand ocular diseases such as diabetic retinopathy and age-related macular degeneration [8–11]. Currently, multiple assays exist for studying choroidal endothelial cells, including the ex vivo choroid sprouting assay, enzymatic digestion-based isolation, and immunoselection methods. Through the foundational work of previous studies, we have gained valuable insights into the isolation and culture of choroidal endothelial cells. The ex vivo choroid sprouting assay, developed by Shao et al. and Tomita et al., has provided a robust model for studying choroidal microvascular angiogenesis, demonstrating the importance of maintaining a physiologically relevant microenvironment [12,13]. Meanwhile, protocols for endothelial cell isolation from various mouse tissues, including the choroid, outlined by Daneman et al., have highlighted effective strategies for obtaining high-purity endothelial cell populations through enzymatic digestion and immunoselection [14]. In addition, several studies have reported robust methods for isolating endothelial cells and stromal vascular fraction (SVF) cells from other vascular beds, including adipose tissue and the lung, using a combination of mechanical dissociation, enzymatic digestion, and magnetic or flow cytometry–based enrichment [15–17].
Building on these methodologies, we have refined our protocol to enhance the efficiency and specificity of primary mouse choroidal endothelial cell (mCEC) culture. By integrating MatrigelTM-based explant culture with CD31+ magnetic-activated cell sorting (MACS), we achieve high endothelial cell purity while preserving key functional properties. Additionally, we have optimized culture conditions to balance EC survival and growth while minimizing contamination from other cell types. Although our approach still has limitations, such as the requirement for fresh tissue and extended culture time, it offers a strategic adjustment and reproducible method for studying choroidal endothelial cells at a molecular level. By learning from these established methodologies and making strategic adjustments, our refined protocol provides an improved approach for investigating choroidal endothelial cell biology, facilitating further studies on choroidal angiogenesis and related diseases.
Materials and reagents
Biological materials
1. 6–8-weeks-old C57BL/6J mice (The Jackson Laboratory, Stk#000664)
2. Endothelial cell (EC) lineage tracing mice (Cdh5Cre; Rosa26-EYFPf/f mice) were generated by crossing Cdh5Cre constitutive mice (The Jackson Laboratory, Stk#006137) with Rosa26-EYFPf/f mice (The Jackson Laboratory, Stk#006148)
Reagents
A. Isolation and culture of mouse choroidal endothelial cells
1. DPBS (Gibco, catalog number: 14190250)
2. MatrigelTM (Corning, catalog number: 354230)
3. Dispase (Corning, catalog number: 354235)
4. EGMTM-2 endothelial cell growth medium (Lonza, catalog number: CC-3162)
5. CD31 MicroBeads, mouse (Miltenyi Biotec, catalog number: 130-097-418)
6. Penicillin-streptomycin (Gibco, catalog number: 15-070-063)
7. FcR blocking reagent, mouse (Miltenyi Biotec, catalog number: 130-092-575)
8. Ketamine (Henry Schein Animal Health, catalog number: 071069)
9. Xylazine (Akorn Pharmaceuticals Corporate, catalog number: 033197)
B. Immunofluorescence staining
1. 4% paraformaldehyde (PFA) in PBS (Santa Cruz Biotechnology, catalog number: sc-281692)
2. 10% normal goat serum (Thermo Scientific, catalog number: 50062Z)
3. ACTA2 antibody (Santa Cruz Biotechnology, catalog number: sc-32251)
4. Alexa Fluor 594-conjugated goat anti-mouse secondary antibody (InvitrogenTM, catalog number: A11032)
5. DAPI and Hoechst nucleic acid stains (InvitrogenTM, catalog number: D1306)
6. VECTASHIELD antifade mounting medium (Vector Laboratories, catalog number: H-1000-10)
7. Tween-20 (MP Biomedicals, catalog number: 11TWEEN201-CF)
8. Triton X-100 (Thermo Scientific, catalog number: A16046.AP)
C. Western blot
1. RIPA buffer (Sigma-Aldrich, catalog number: R0278)
2. Phosphatase inhibitor (Sigma-Aldrich, catalog number: 4906845001)
3. Protease inhibitor cocktail (Sigma-Aldrich, catalog number: 05892970001)
4. PierceTM BCA Protein Assay kit (Fisher Scientific, catalog number: 23225)
5. Recombinant mouse TGF-beta 2 protein (R&D Systems, catalog number: 7346-B2-005)
6. ACTA2 antibody (Santa Cruz Biotechnology, catalog number: sc-32251)
7. SM22α antibody (Abcam, catalog number: ab14106)
8. COL1 antibody (Novus Biologicals, catalog number: NB600-408)
9. CD31 antibody (Santa Cruz Biotechnology, catalog number: 376764)
10. β-actin antibody (Santa Cruz Biotechnology, catalog number: sc-47778)
11. Anti-mouse IgG HRP-linked antibody (Cell Signaling Technology, catalog number: 7076S)
12. Anti-rabbit IgG HRP-linked antibody (Cell Signaling Technology, catalog number: 7074S)
13. 10× tris buffered saline (TBS) (Bio-Rad, catalog number: 1706435)
14. Immobilon Forte Western HRP substrate (MilliporeSigma, catalog number: CHM01S430)
15. Non-fat milk (Lab Scientific, catalog number: NC9121673)
Solutions
1. PBST buffer (see Recipes)
2. Permeabilization buffer (see Recipes)
3. TBST buffer (see Recipes)
4. 5% non-fat milk (blocking buffer) (see Recipes)
Recipes
1. PBST buffer
Store at room temperature.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS (1×) | 1× | 500 mL |
| Tween-20 | 0.05% (v/v) | 250 μL |
| Total | n/a | 500 mL |
2. Permeabilization buffer
Store at room temperature.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS (1×) | 1× | 500 mL |
| Triton X-100 | 0.05% (v/v) | 250 μL |
| Total | n/a | 500 mL |
3. TBST buffer
Store at room temperature.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| TBS (10×) | 1× | 100 mL |
| Tween-20 | 0.05% (v/v) | 500 μL |
| H2O | n/a | 900 mL |
| Total | n/a | 1,000 mL |
4. 5% non-fat milk (blocking buffer)
Prepare fresh before use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| TBST (Recipe 3) | 1× | 30 mL |
| Non-fat milk | 5% (w/v) | 1.5 g |
| Total | n/a | 30 mL |
Laboratory supplies
1. 24-well plate (Corning, catalog number: 3524)
2. 40 μm cell strainer (Corning, catalog number: 352340)
3. 35 mm dish (Corning, catalog number: 430165)
4. 8-well cell culture slides (Falcon, catalog number: 354118)
5. 6-well plate (Corning, catalog number: 3516)
6. Microscope slides (Fisher Scientific, catalog number: 12-550-15)
7. Cytiva AmershamTM HybondTM PVDF membranes (Fisher Scientific, catalog number: 45-004-021)
8. ProfessionalTM Kimtech ScienceTM KimwipesTM (Fisher Scientific, catalog number: 06-666)
9. AxygenTM MaxyClear snaplock 1.5 mL microtubes (Fisher Scientific, catalog number: 14-222-155)
10. FalconTM 15 mL conical centrifuge tubes (Fisher Scientific, catalog number: 14-959-53A)
11. CorningTM centrifuge 50 mL tubes (Fisher Scientific, catalog number: 05-526B)
12. LS columns (Miltenyi Biotec, catalog number: 130-042-401)
13. Iris forceps, curved (World Precision Instruments, catalog number: 15915)
14. Dumont Dumoxel tweezers (World Precision Instruments, catalog number: 14188)
15. Dumont titanium forceps (World Precision Instruments, catalog number: 14187)
16. Noyes micro scissors (World Precision Instruments, catalog number: 500228)
Equipment
1. Cell incubator (Thermo Scientific, catalog number: 13-998-252L7)
2. Centrifuge (Eppendorf, catalog number: 5430R)
3. ChemiDocTM imaging system (Bio-Rad, catalog number: 12003153)
4. Inverted Routine Eclipse TS2 microscope for cell morphology (Nikon, catalog number: 3374842)
5. Zeiss 780 inverted confocal for immunofluorescence (Zeiss, catalog number: 420650-9901-000)
6. MACS® MultiStand (Miltenyi Biotec, catalog number: 130-042-303)
7. QuadroMACSTM Separator and Starting kits (Miltenyi Biotec, catalog number: 130-091-051)
8. Microscope for phase contrast photos of individual explants (Leica, model: DMi8)
9. TC20 automated cell counter (Bio-Rad, catalog number: 1450102)
Software and datasets
1. Image J software (National Institutes of Health; RRID: SCR_003070)
2. Zeiss software (ZEN lite)
3. Image Lab 6.1 (Bio-Rad)
Procedure
文章信息
稿件历史记录
提交日期: Mar 26, 2025
接收日期: May 22, 2025
在线发布日期: Jun 5, 2025
出版日期: Jun 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yang, Q., Cai, Y., Ma, Q. and Huo, Y. (2025). Primary Mouse Choroidal Endothelial Cell Culture. Bio-protocol 15(12): e5355. DOI: 10.21769/BioProtoc.5355.
- Yang, Q., Cai, Y., Ma, Q., Xiong, A., Xu, P., Zhang, Z., Xu, J., Zhou, Y., Liu, Z., Zhao, D., et al. (2024). Inactivation of adenosine receptor 2A suppresses endothelial-to-mesenchymal transition and inhibits subretinal fibrosis in mice. Sci Transl Med. 16(737): eadk3868. https://doi.org/10.1126/scitranslmed.adk3868
分类
细胞生物学 > 细胞分离和培养 > 细胞分离
细胞生物学 > 细胞分离和培养 > 细胞生长
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link