- EN - English
- CN - 中文
Protocol of Whey Protein Isolate–Based Microgel Targeted Delivery in Mouse Kidney
基于乳清蛋白分离物微凝胶的小鼠肾脏靶向递送实验方案
发布: 2025年06月20日第15卷第12期 DOI: 10.21769/BioProtoc.5347 浏览次数: 1953
评审: Olga KopachAnonymous reviewer(s)
Abstract
Every year, there is an increase in the number of cases of chronic kidney disease, and a delay in the initiation of adequate treatment can lead to kidney failure, which requires regular dialysis or transplantation. Intensive systemic therapy used to treat kidney diseases often has a negative impact on other weakened organs, making it crucial to ensure targeted delivery of medications directly to the kidneys and to minimize systemic side effects. In order to reduce the toxicity of medications and decrease dosages, innovative delivery methods are being developed, such as micro-sized targeted delivery systems, which ensure highly effective distribution of encapsulated drugs directly within the organs. In a recent article, we presented innovative emulsified microgels stabilized with whey protein isolate (WPI), specifically designed for targeted drug delivery to the kidneys. Our stability studies revealed that these microgels start to degrade after 72 h, with this degradation exhibiting a time-dependent profile. Furthermore, intravenous administration of the microgel suspension through the tail vein showed significant selective accumulation in both the liver and kidneys over a duration of 5 days. As part of our research, we present the protocol for synthesizing emulsion microgels derived from whey protein isolate. This article provides a comprehensive overview of the procedures for precursor preparation, along with an in-depth investigation of the emulsion system's stability over time. The protocol also includes the injection of an emulsion microgel suspension into the tail vein of mice, enabling the evaluation of their biocompatibility and potential therapeutic efficacy. This protocol outlines the precautions and important nuances that should be considered at each stage of the experiment.
Key features
• This protocol provides detailed instructions on the synthesis of fluorescently labeled emulsion microgels using mucoadhesive whey protein isolate, allowing the replication of this process.
• The protocol describes in detail how to prepare samples for confocal microscopy.
• We are exploring a method to study the stability of emulsion microgels by analyzing the size of emulsion droplets using optical microscopy.
• We provide guidance on the administration of suspension into the tail vein of mice, followed by visualization of the microgels' body biodistribution.
Keywords: Emulsion microgels (EM) (乳液微凝胶(EM))Graphical overview
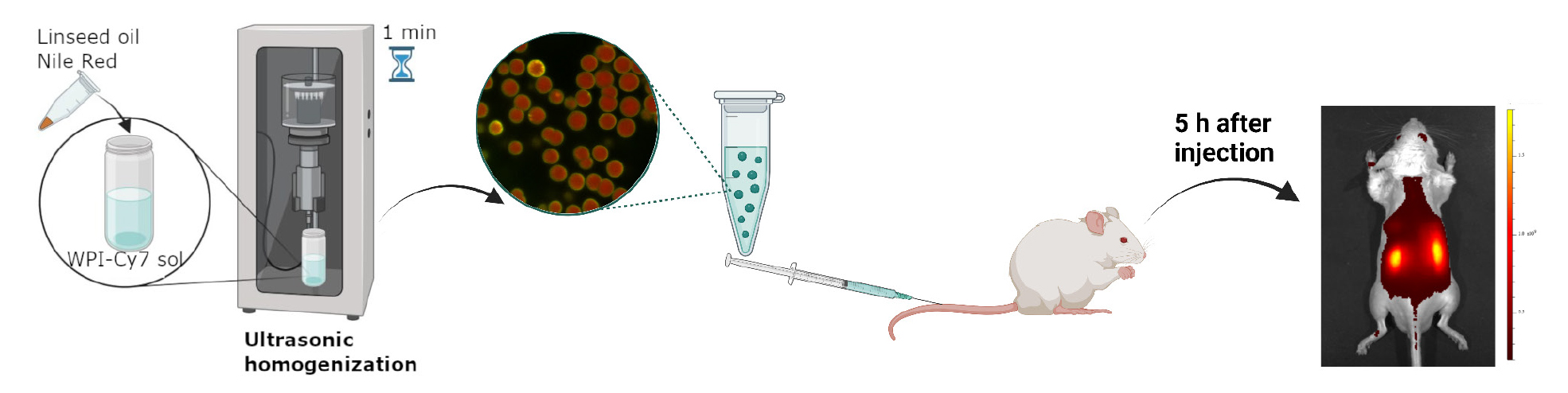
Background
The prevalence of chronic kidney disease continues to steadily increase each year [1,2]. Delayed initiation of adequate therapy can lead to kidney failure [3,4]. Often, intensive systemic therapy causes complications in other weakened organs and systems, highlighting the need for targeted drug delivery directly to the affected organ to minimize systemic side effects of treatment. The method of administering drugs through the renal artery has the potential to concentrate therapeutic agents in the cargo, which could greatly enhance the treatment of chronic kidney disorders. However, this approach carries risks such as vascular damage, aortic blockage, and the need for highly trained personnel.
To address this issue and to reduce the necessary therapeutic doses, alternative addressing methods are being developed, such as micro-sized targeted delivery systems [5,6]. These systems can effectively regulate the distribution of encapsulated drugs in the organs, opening new horizons in the treatment of chronic kidney disease.
Currently, emulsion systems are actively being studied as a promising treatment for diseases of the genitourinary system [7]. One of the main advantages of these systems for targeted drug delivery is their ability to provide long-term release of both hydrophobic and hydrophilic drugs [7]. This is due to the presence of both aqueous and oil phases in the system.
Modification of emulsions with mucoadhesive components allows an increase in targeting of the drug delivery system, their retention on the mucous surface of organs, and, at the same time, the localization, safety, and effectiveness of the administered drug. The use of mucoadhesive emulsions is gaining popularity due to their unique combination of properties that make them ideal for delivering drugs directly to the mucosal surfaces. In our study, we use the naturally derived mucoadhesive component whey protein isolate (WPI). WPI, along with its efficient mucosal specificity, provides the formation of the hydrogel shell at the oil surfaces upon the ultrasonically stimulated denaturation. In this way, the emulsion microgel system is formed.
Recently, we described the protocol for synthesizing emulsion microgels with high mucosal adhesion properties to the urothelium of the bladder provided by the mucoadhesive component, WPI [8]. We provided precautions and nuances that should be paid attention to at each stage of the experiment. During the study, the main physico-chemical parameters of microgels, such as filling capacity and drug release rate, were studied. The processes of drug release, both from the oil core of microgels (hydrophobic drug) and from the hydrogel protein shell (hydrophilic drug), strongly depend on the ionic strength of the medium in which microgels are incubated [8–10]. It has been shown that the ionic strength affects the degradation rate of protein hydrogel shells and, thus, the overall stability of microgels. This, in turn, affects the drug release behavior. The high ionic strength of the medium causes the high hydrophobicity of the protein molecules in the shell, which, accordingly, increases the stability of the microgels. The stability of microgels in artificial urine (0.3 M) is higher than in saline solution (0.15 M). Correspondingly, in artificial urine, the drug release from microgels demonstrates a more prolonged behavior, as compared with saline 0.15 M NaCl. The amount of hydrophilic drug released from the protein shell reaches 45% at 72 h of incubation in artificial urine [8]. The biodistribution of emulsion microgels in vivo after intravesical administration showed that local delivery ensures effective accumulation of carriers in the bladder due to the high adhesive properties of the protein on the surface of the microgels.
Due to the possibility of varying the synthesis conditions to produce microgels with precisely specified physical and chemical properties, such as particle size, surface area, zeta potential, and drug loading efficiency, emulsion microgels have been studied as a potential drug delivery system for the kidneys [11]. Intravenous delivery of these microgels resulted in a long-term accumulation (at least 5 days) of these particles in the liver and kidneys, indicating a high efficiency of biodistribution and targeting to the target organ. The experimental conditions for synthesizing these microgels allow for the creation of particles with exactly the desired characteristics. The developed microgels provide a customized therapeutic approach to the treatment of kidney diseases, with reduced side effects. This opens up new possibilities for the systemic treatment of the urinary system.
This protocol provides detailed instructions on the synthesis, preparation, and research of fluorescently labeled emulsion microgels based on whey protein isolate. It allows researchers to accurately replicate these procedures and achieve the desired results.
The protocol includes the following steps:
1. Synthesis of fluorescently labeled microgels: This involves the preparation of a mixture of whey protein isolate and a fluorescent dye, which is then processed to create the microgel structure.
2. Preparation of samples for confocal microscopy: The microgels are prepared for analysis by confocal microscopy, which helps to visualize their structure and distribution.
3. Optical microscopy analysis: The stability of the microgels is assessed using optical microscopy, specifically focusing on the size and distribution of the emulsion droplets within the microgel.
4. In vivo imaging: A suspension of the microgels is injected into the tail vein of a mouse, allowing for the visualization of the distribution of the fluorescent dye signal in the animal's body using fluorescence imaging.
Overall, this protocol provides a comprehensive guide for researchers interested in studying fluorescently labeled emulsion microgels using whey protein as a basis. It ensures accurate and efficient sample preparation, making it a valuable tool for further research in this field.
Materials and reagents
Reagents
1. Linseed oil OLEOS (Russia) (unrefined cold pressed linseed oil, refractive index n20/D 1.4795, density 0.93 g/mL at 25 °C, commercial use)
Note: It is possible to use linseed oil from another manufacturer. However, it is worth paying attention to its properties (the refractive index n20/D of 1.4795 and the density of 0.93 g/mL at 25 °C).
2. Whey protein isolate (WPI) California Gold Nutrition (USA), iHerb, https://www.californiagoldnutrition.com/products/california-gold-nutrition-sport-whey-protein-isolate-unflavored-5-lb-2-27-kg-76479 (27 g of protein, 6.2 g of BCAAs, 4.7 g of glutamic acid, low lactose; lactose is used as an excipient, which is not involved in the formation of emulsion microgels; no additives or flavor enhancers)
Note: It is important to pay attention to the content of additional proteins in the WPI composition (such as BCAAs and glutamic acid) since this can affect the properties of the resulting emulsion microgels. It is also worth avoiding the presence of flavor enhancers and additives in the WPI composition.
3. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number S9888)
4. Cyanine 7 NHS-ester (Cy7), analytical reagent grade; reagent for research and development. Quality control: NMR 1H, HPLC–MS (95%) (Lumiprobe, catalog number 65020)
5. Phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number P4417)
6. Dimethyl sulfoxide (DMSO) (EcoChemAnalyt, Russia, CAS: 67-68-5)
7. Sodium hydroxide (NaOH) (EcoChemAnalyt, Russia, State Standard 4328-77, CAS: 1310-73-2)
8. Nile red (Sigma-Aldrich, catalog number: 72485)
9. Milli-Q water (Millipore filtration system) (used in all experiments)
10. Zoletil (Virbac, catalog number: L042-00118-31/00014469)
11. Xylazine (Alfasan International, catalog number: 528-3-7.16-3256№PBI-3-8.0/03450)
Solutions
1. 5% WPI aqueous solution (see Recipes)
2. Saline, 0.9% NaCl solution (see Recipes)
3. 5 mg/mL Cy7 solution (see Recipes)
4. 1 M NaOH solution (see Recipes)
5. PBS solution (0.1 M, pH 8.3) (see Recipes)
Recipes
1. 5 % WPI (w/v) aqueous solution
| Reagent | Final concentration | Amount |
|---|---|---|
| WPI | 5 % | 500 mg |
| Saline 0.9% NaCl solution (Recipe 2) | 0.9% (w/v) | 10 mL |
| Total | n/a | 10 mL |
Caution: The solution must be mixed thoroughly. Wait until the foam disappears before use.
Note: For the conjugation procedure, protein solutions are prepared in PBS (pH 8.3) in equivalent amounts.
2. Saline, 0.9% NaCl (w/v) solution
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 0.9% (w/v) | 9 g |
| H2O | n/a | 1 L |
| Total | n/a | 1 L |
3. 5 mg/mL Cy7 solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Cy7 | 5 mg/mL | 5 mg |
| DMSO | n/a | 1 mL |
| Total | n/a | 1 mL |
Note: Due to the difficulty in weighing a 5 mg sample, it is advisable to prepare a larger quantity of the Cy7, which necessitates a larger volume of DMSO to create the final solution with the desired Cy7 concentration of 5 mg/mL. To achieve this, you must proportionally increase the mass of the Cy7 and the volume of DMSO. For instance, weigh out 25 mg of Cy7 and then add DMSO until the volume reaches 5 mL. This will result in a final Cy7 solution of 5 mg/mL, in a volume of 5 mL.
4. 1 M NaOH solution (100 mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| NaOH | 1 M | 0.4 g |
| H2O | n/a | 100 mL |
| Total | n/a | 100 mL |
5. PBS solution (0.1 M, pH 8.3)
| Reagent | Final pH | Amount |
|---|---|---|
| PBS | pH 7.2 | 1 tablet |
| H2O | n/a | 200 mL |
| Total | n/a | 200 mL |
Note: Adjust the pH of the resulting solution to 8.3 using NaOH 1 M.
Laboratory supplies
1. Centrifuge tubes with flat cap, 15 mL (JetBioFil, catalog number: CFT550150)
2. Centrifuge tubes with flat cap, 50 mL (JetBioFil, catalog number: CFT500500)
3. Microcentrifuge tubes, 1.5 mL (JetBioFil, catalog number: CFT000015)
4. Microcentrifuge tubes, 2.0 mL (JetBioFil, catalog number: CFT000020)
5. Laboratory glass jar 100 mL, with divisions, with screw lid, dark glass (MiniMed, catalog number: 10007205)
6. Vial 2 mL, dark glass (ALWSCI Technologies, catalog number: C0001177)
7. Vial 10 mL, clear glass, 22 mm × 52 mm (ALWSCI Technologies, catalog number: C0000053)
8. Dialysis bag M-Cel, pore diameter 14 kDa (Viscase, catalog number: 2141-1425)
9. Dialysis bag clamp (Scienova GmbH, catalog number: 40329)
10. Laboratory beaker, clear glass, 2 L (MiniMed, catalog number: 10003807)
11. Glass for microslides (MiniMed, catalog number: 12003421)
12. Cover glass for microslides (MiniMed, catalog number: 12003309)
13. Syringe 1 mL (BD Micro-Fine Plus, catalog number: 320935)
14. Pipette microtips, 2–20 μL (JetBioFil, catalog number: PPT100020)
15. Pipette microtips, 10–200 μL (JetBioFil, catalog number: PPT000200)
16. Pipette microtips, 100–1,000 μL (JetBioFil, catalog number: PPT000000)
Equipment
1. Bandelin Sonopuls HD 2070 homogenizer at a frequency of 20 kHz and a power density of 1 W/cm2 (Germany)
2. Magnetic stirrer (IKA, model: 0025004132)
3. Multifunctional refrigerated centrifuge (Eppendorf, model: 5810R)
4. Inverted confocal microscope (Leica Microsystems, model: Leica TCS SP8 X)
5. Inverted microscope, 40× objective (Olympus, model: IX73)
6. Single-channel variable volume dispenser 100–1,000 mL, 10–100 mL, 20–200 mL, 5–50 mL, and 1,000–5,000 mL (Thermo Fisher Scientific)
7. IVIS SpectrumCT imaging system (PerkinElmer, Waltham, MA, USA)
8. Water purification system for ultrapure water (Merck Millipore, model: Milli-QTM Advantage A10TM)
Software and datasets
1. ImageJ software 1.51j8 (National Institutes of Health, USA) (the open-source version is available online for free for download)
2. OriginPro 2018 SR1 (OriginLab Corporation, USA)
3. Excel 2019 (Microsoft Corporation, USA)
4. LAS X Life Science Microscope Software (Leica Microsystems, Germany)
5. Living Image 4.7.3 software (PerkinElmer, USA)
Procedure
文章信息
稿件历史记录
提交日期: Mar 19, 2025
接收日期: May 13, 2025
在线发布日期: May 30, 2025
出版日期: Jun 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Mayorova, O. A., Saveleva, M. S., Terentyeva, D. A., Gusliakova, O. I. and Sindeeva, O. A. (2025). Protocol of Whey Protein Isolate–Based Microgel Targeted Delivery in Mouse Kidney. Bio-protocol 15(12): e5347. DOI: 10.21769/BioProtoc.5347.
分类
生物工程 > 生物医学工程 > 药物递送
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













