- EN - English
- CN - 中文
A Novel Protein Purification Approach Using Elastin-Like Polypeptides (ELP) With His-Tag Assistance
结合His标签辅助的弹性蛋白样多肽(ELP)新型蛋白纯化方法
发布: 2025年06月20日第15卷第12期 DOI: 10.21769/BioProtoc.5342 浏览次数: 3115
评审: Alessandro DidonnaNuttavut KosemRupam GhoshAnonymous reviewer(s)
Abstract
Protein purification is essential for drug development, antibody production, and structural biology. We propose a cost-effective chromatography method using elastin-like polypeptide (ELP) as an aggregating core. In this approach, a chilled (target protein)-ELP fusion is loaded onto an immobilized metal affinity chromatography (IMAC) column equilibrated with low-salt buffer. Impurities are removed with warm high-salt buffer washes. Warming the column above the ELP’s transition temperature (Tm) triggers ELP aggregation, physically trapping the target protein between beads. Subsequent stringent washing (high salt/imidazole) eliminates residual contaminants. Finally, cooling with cold low-salt buffer reverses aggregation, eluting the purified target protein. This method eliminates the need for advanced chromatography systems while achieving high purity through dual mechanisms: (1) IMAC affinity and (2) temperature-dependent physical capture. The ELP’s reversible phase transition offers a simplified yet efficient purification platform, particularly valuable for lab-scale production of challenging proteins.
Key features
• This protocol requires an elastin-like polypeptide tag at the C-terminus of the target protein.
• This protocol requires a His-Tag at the N-terminus of the target protein.
• This protocol requires the use of colored/chromogenic proteins to enable real-time visual monitoring of chromatographic progression.
• This protocol yields a highly pure protein by manually operating a Ni-bound resin at two different temperatures.
Keywords: Aggregation (聚集)Graphical overview
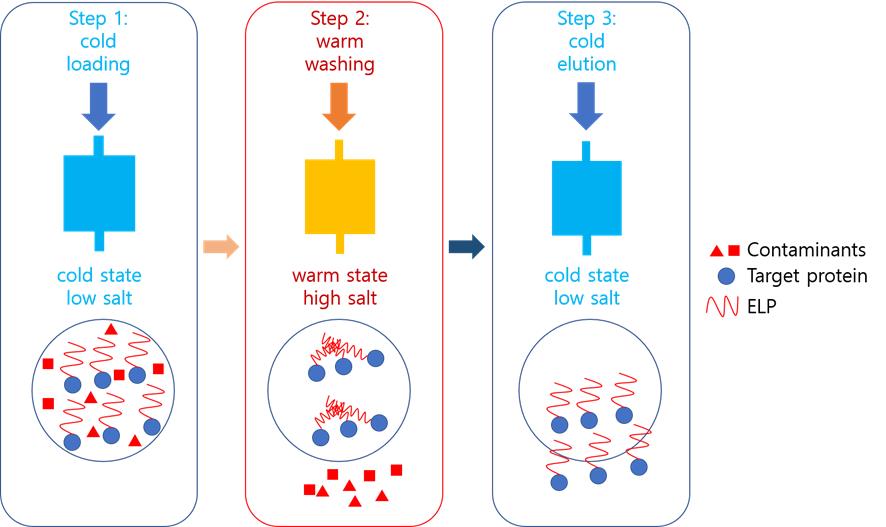
Background
Protein purification is pivotal for various uses, especially in structure-based drug discovery, where high purity levels are crucial [1,2]. Recent advancements in molecular biology techniques, such as PCR, have spurred the production of recombinant proteins, allowing for their large-scale production and purification [3,4]. These proteins have diverse applications, including vaccines, therapeutics, and diagnostics [4]. Escherichia coli is often the organism of choice for producing recombinant proteins due to its rapid growth, cost-effectiveness, and high yield [5–8]. By introducing genes into E. coli via plasmids, controlled protein expression can be achieved upon induction.
Protein purification methods, such as precipitation or chromatography, are employed based on the protein's characteristics. Affinity chromatography is particularly favored for its efficiency in purifying recombinant proteins [9]. Various strategies have been developed to enhance this process. Fusion proteins with tags like glutathione S-transferase (GST), maltose binding protein (MBP), or polyhistidine are common as they aid in production and purification [10–12]. Signal peptides can facilitate periplasmic secretion, reducing contamination [13,14]. Aggregation tags induce protein precipitation for easier purification. GST or MBP fusion proteins are typically purified using glutathione or maltose resins, while polyhistidine tags use resins with immobilized metal ions. Periplasmic secretion is advantageous due to fewer contaminating proteins compared to cytoplasmic secretion. Aggregation tags manipulate factors like temperature or salt concentration for purification [15]. The elastin-like polypeptide (ELP) uses inverse temperature cycling (ITC), which requires high-temperature centrifugation, presenting challenges for low-temperature centrifuges [16].
This study introduces a novel method called aggregation-assisted chromatography for purifying fusion proteins with both precipitation and polyhistidine tags. The technique employs an immobilized metal affinity chromatography (IMAC) column, with adjustments in temperature and salt concentration to achieve high purity. By controlling the buffer temperature, this method allows for high-purity manual purification without the need for sophisticated chromatography equipment. Although switching the order of the two tags may not significantly affect overall protein expression, it is possible that the ELP, due to its intrinsically disordered nature, could influence the folding of the downstream protein.
Materials and reagents
Biological materials
1. E. coli strain specialized for protein production, Rosetta2(DE3)pLysS (Merck, catalog number: 71403)
2. DH5α (Enzyomics, catalog number: CP011)
3. pVP65KR-SacB-I48-based plasmid harboring a desired gene [17–20]. SnapGene files of all plasmids will be provided upon request
Reagents
1. Imidazole (Duksan, catalog number: 7097)
2. Sodium phosphate, dibasic (Na2HPO4) (Duksan, catalog number: 1490)
3. Sodium phosphate, monobasic (NaH2PO4) (Duksan, catalog number: 3989)
4. Sodium chloride (NaCl) (Duchefa Biochemie, catalog number: T0520.1000)
5. Trypton (Duchefa Biochemie, catalog number: T1332.0500)
6. Yeast extract (Duchefa Biochemie, catalog number: T1333.0500)
7. IPTG (Gold Biotechnology, catalog number: I2481C)
8. Triton X-100 (MP Biomedicals, catalog number: 02194854-CF)
9. SfaAI (ThermoFisher, catalog number: ER2091)
10. MssI (ThermoFisher, catalog number: ER1342)
11. Rapid DNA Ligation kit (ThermoFisher, catalog number: K1422)
12. Gel Extraction kit (Cosmogenetech, catalog number: CMG0112)
Solutions
1. LB medium (see Recipes)
2. 1 M IPTG (see Recipes)
3. 0.2 M sodium phosphate, dibasic (see Recipes)
4. 0.2 M sodium phosphate, monobasic (see Recipes)
5. PBS (see Recipes)
6. Binding buffer (see Recipes)
7. Washing buffer (see Recipes)
8. Elution buffer (see Recipes)
9. 20% Triton X-100 (see Recipes)
Recipes
1. LB medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Trypton | 1% | 10 g |
| Yeast extract | 0.5% | 5 g |
| NaCl | 1% | 10 g |
| Total | n/a | 1,000 mL |
Sterilize the medium by autoclaving at 121 °C for 20 min.
2. 1 M IPTG
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| IPTG | 1 M | 2.38 g |
| Total | n/a | 10 mL |
Aliquot the solution into 1mL portions and store at -20 °C.
3. 0.2 M sodium phosphate, dibasic
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 0.2 M | 28.4 g |
| Total | n/a | 1,000 mL |
4. 0.2 M sodium phosphate, monobasic
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaH2PO4 | 0.2 M | 24.0 g |
| Total | n/a | 1,000 mL |
5. PBS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.2 M sodium phosphate, dibasic | 10 mM | 25 mL |
| 0.2 M sodium phosphate, monobasic | 10 mM | 25 mL |
| NaCl | 150 mM | 58.44 g |
| Total | n/a | 1,000 mL |
Sterilize the buffer by autoclaving at 121 °C for 20 min.
6. Binding buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.2 M sodium phosphate, dibasic | 10 mM | 25 mL |
| 0.2 M sodium phosphate, monobasic | 10 mM | 25 mL |
| NaCl | 150 mM | 58.44 g |
| Imidazole | 10 mM | 0.68 g |
| Total | n/a | 1,000 mL |
7. Washing buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.2 M sodium phosphate, dibasic | 10 mM | 25 mL |
| 0.2 M sodium phosphate, monobasic | 10 mM | 25 mL |
| NaCl | 150 mM | 58.44 g |
| Imidazole | 250 mM | 17.02 g |
| Total | n/a | 1,000 mL |
8. Elution buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.2 M sodium phosphate, dibasic | 10 mM | 25 mL |
| 0.2 M sodium phosphate, monobasic | 10 mM | 25 mL |
| NaCl | 150 mM | 58.44 g |
| Imidazole | 500 mM | 34.04 g |
| Total | n/a | 1,000 mL |
9. 20% Triton X-100
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Triton X-100 | 20% (v/v) | 20 mL |
| Total | n/a | 100 mL |
Laboratory supplies
1. 3 L baffled polycarbonate Erlenmeyer flasks (Corning, catalog number: 431253)
2. 50 mL centrifuge tubes (Corning, catalog number: CLS430829)
3. 10 μL pipette tips (Axygen, catalog number: T-300-L)
4. 200 μL pipette tips (Axygen, catalog number: T-200-C)
5. 1,250 μL pipette tips (Axygen, catalog number: MTX-1250-C)
6. 20 mL syringe with a Luer lock tip (Beckton Dickinson, catalog number: 302830)
7. 1/16” male/Luer female adaptor (Cytiva, catalog number: 18111251)
Equipment
1. High-speed centrifuge (Hanil, model: Supra R17)
2. Pipetman (Gilson, catalog number: F167350)
3. Racks (Korea Ace Scientific, catalog number: KA.UB33-27)
4. Water bath (Branson, catalog number: CPX1800H-E)
5. Shaking incubator (Vision Scientific, catalog number: VS-37SI)
6. Deep freezer (-80 °C) (Nihon Freezer, model: MY BIO)
7. Ultrasonic processor (Sonics, model: VCX 750)
8. HisTrap HP 5 mL column (Cytiva, catalog number: 17524801)
9. UV-Vis spectrophotometer (Scinco, model number: S-3100)
Procedure
文章信息
稿件历史记录
提交日期: Mar 11, 2025
接收日期: May 14, 2025
在线发布日期: May 30, 2025
出版日期: Jun 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Chae, Y. K. and Shin, H. B. (2025). A Novel Protein Purification Approach Using Elastin-Like Polypeptides (ELP) With His-Tag Assistance. Bio-protocol 15(12): e5342. DOI: 10.21769/BioProtoc.5342.
分类
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













