- EN - English
- CN - 中文
Murine Osteoblast and Osteoclast Co-culture on Demineralized Bone Paper for Bone Remodeling
在脱矿骨纸上共培养小鼠成骨细胞与破骨细胞用于研究骨重塑
(*contributed equally to this work) 发布: 2025年06月05日第15卷第11期 DOI: 10.21769/BioProtoc.5339 浏览次数: 2235
评审: Valérian DORMOYAnonymous reviewer(s)
Abstract
Continuous and balanced bone remodeling is essential for maintaining mechanical integrity, mineral homeostasis, and hematopoiesis. Dysregulated bone metabolism develops pathological conditions, such as osteoporosis and bone metastasis. Functional and analytical recapitulation of bone remodeling in vitro is critical for advancing our understanding of bone mineral metabolism, disease mechanisms, and drug development. However, conventional models fail to replicate the essential complexity of the bone extracellular matrix (ECM) and the dynamic interplay between bone-forming osteoblasts and bone-resorbing osteoclasts. Recently, we developed an osteoid-mimicking demineralized bone paper (DBP) by thin-sectioning demineralized bovine compact bone matrix. DBP supports osteoblastic mineral deposition and the subsequent transition to bone-lining cells. When co-cultured with bone marrow mononuclear cells under biochemical stimulation, osteoblasts shift their regulatory secretion profiles and effectively induce osteoclastogenesis. The semi-transparent nature of DBP, combined with primary osteogenic cells retrieved from DsRed and eGFP reporter mice, enables longitudinal fluorescent monitoring of these multicellular processes and quantitative analysis. In this protocol, we describe the methods for DBP generation, reconstituting mineralized bone tissue complexity with osteoblasts, and recapitulating the bone remodeling cycle through bone marrow monocytes co-culture under biochemical stimulation, offering a useful platform for the related and broader research community.
Key features
• DBP supports in vivo–relevant osteoblastic mineral deposition and transition to lining cells.
• DBP supports in vivo–relevant recapitulation of osteoblast–osteoclast-driven bone remodeling.
• DBP supports longitudinal fluorescent monitoring of multicellular processes and quantification.
Keywords: Demineralized bone paper (脱矿骨纸)Graphical overview
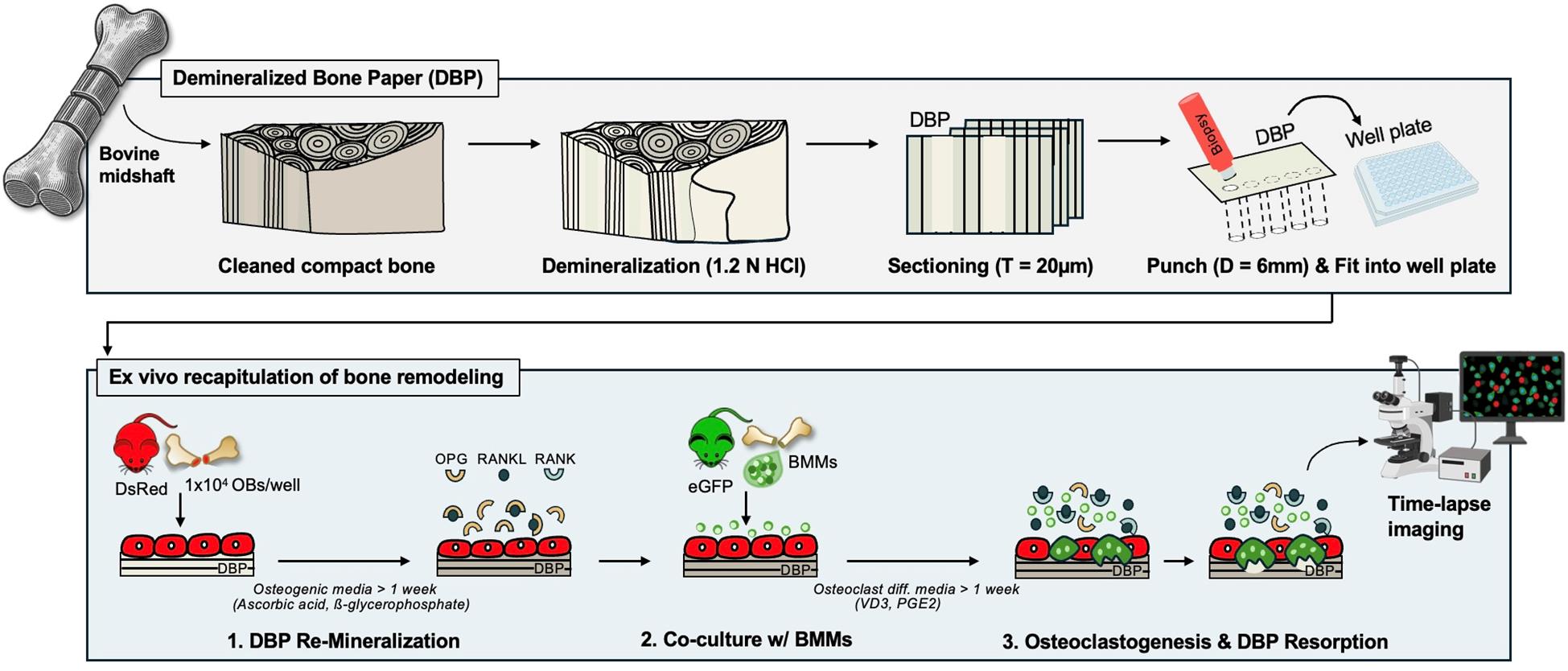
Background
Continuous bone remodeling is essential for maintaining mechanical integrity, mineral homeostasis, and blood formation [1,2]. This process is driven by the coupled actions of bone-resorbing osteoclasts and bone-forming osteoblasts under the spatiotemporal regulation of stimulative and suppressive molecules. Receptor activator of nuclear factor κB ligand (RANKL) is a key stimulatory molecule that induces the differentiation of bone marrow monocytes into multinucleated osteoclasts. Osteoprotegerin (OPG), a decoy receptor for RANKL, serves as a key suppressive molecule that prevents bone remodeling by blocking RANKL-RANK receptor interactions [3,4]. While these multicellular and molecular processes are well established in animal models [5,6] and clinical studies [7,8], their in vitro recapitulation remains a significant challenge.
One important consideration in developing bone tissue models outside the body is the unique hierarchical organization of the bone extracellular matrix (ECM), which consists of collagen fibers and hydroxyapatite crystals at multiple length scales [9]. This mature lamellar bone structure is continuously broken down and rebuilt throughout life. During this process, osteoblasts first deposit a structural collagen matrix, known as osteoid, which then gradually mineralizes to form a lamellar mineralized collagen matrix [10,11]. We hypothesized that mimicking the osteoid-state bone ECM would be a promising strategy to reproduce bone formation and remodeling in vitro. To test this hypothesis, we created demineralized bone paper (DBP) by thin sectioning demineralized bovine compact bone in 2021 [12]. DBP preserves the collagen structure of mature lamellar bone, and based on the sectioning direction, the collagen organization varies significantly [13]. DBP provides semi-transparency for microscopic imaging and mechanical durability for easy experimental handling. The electrostatic charges of the collagen matrix facilitate stable adhesion to tissue culture plastic surfaces [12–15].
So far, we have demonstrated the enabling features of DBP for in vitro recapitulation of bone tissue complexity and remodeling processes with unprecedented analytical power by using primary cells derived from eGFP and DsRed reporter mice. DBP supports rapid and structurally organized mineral deposition by osteoblasts. These osteoblasts then acquire resting-state bone-lining cell phenotypes, including high OPG and low RANKL secretion. Biochemical stimulation of bone-lining cells shifts their OPG and RANKL secretion profiles, activating osteoclastogenesis in co-cultured bone marrow monocytes (BMCs) and inducing subsequent mineral resorption. Upon withdrawal of chemical stimulants, secretion profiles gradually revert to a resting state, and osteoclasts undergo fission [12,14]. The semi-transparent DBP, combined with genetically labeled osteogenic and hematopoietic cells, enables longitudinal fluorescent monitoring of these multicellular processes [13,15]. In this protocol paper, we compile the related methodological sections for (i) DBP preparation, (ii) DBP remineralization with primary murine osteoblasts, and (iii) the recapitulation of osteoclastogenesis via co-culture of primary murine BMCs under biochemical stimulation.
Materials and reagents
Biological materials
Animals
1. DsRed mice (Jackson Laboratory, catalog number: 006051)
2. eGFP mice (Jackson Laboratory, catalog number: 003291)
Reagents
1. Chloroform (Fisher Chemical, catalog number: C607-4)
2. Methanol (Fisher Chemical, catalog number: A454-4)
3. Hydrochloric acid (HCl) (Fisher Chemical, catalog number: A144-212)
4. Optimal cutting temperature (OCT) compound (Fisher Healthcare, catalog number: 23-730-571)
5. NucBlue Live ReadyProbes (Invitrogen, catalog number: R37605) and Hoechst33342 (Invitrogen, catalog number: H1399)
6. Fluorescent 5-FAM conjugated collagen hybridized peptide (CHP) (3Helix, catalog number: FLU300)
7. Collagenase (Life Technologies, catalog number: 10.17101015)
8. Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F0926-500 mL)
9. Penicillin and streptomycin (PS) (Gibco, catalog number: 15070-063)
10. α-MEM (Gibco, catalog number: 12000-022)
11. 10× Phosphate-buffered saline (PBS) (Fisher Bioreagents, catalog number: BP399500)
12. Sodium bicarbonate (NaHCO3) (Fisher Chemical, catalog number: S233-3)
13. β-glycerophosphate (Sigma, catalog number: G5422-25G)
14. L-ascorbic acid 2-phosphate trisodium salt, 25 G (Wako Chemicals, catalog number: 323-44822)
15. Alkaline phosphatase (ALP) detection kit (Sigma, catalog number: 86C)
16. Vitamin D (VD3) (CAYMAN Chemical Company, catalog number: 71820)
17. Prostaglandin E2 (PGE2) (Cayman Chemical Company, catalog number: 14010)
18. 4% Paraformaldehyde (Thermo Fisher Scientific, catalog number: J19943.K2)
19. Alexa Fluor 488 phalloidin (Invitrogen, catalog number: A12379)
20. Calcein (MP, catalog number: 195087)
21. Alizarin Red S (Acros Organics, catalog number: 400481000)
22. Tartrate-Resistant Acid Phosphatase (TRAP) detection kit (Sigma-Aldrich, catalog number: 387A)
23. Mouse OPG ELISA (R&D Systems, catalog number: DY459)
24. Mouse RANKL ELISA (R&D Systems, catalog number: DY462)
25. Ethanol (Decon Laboratories Inc., catalog number: 2705)
26. Acetic acid (Fisher Scientific, catalog number: A38SI-212)
Solutions
1. 1.2 N HCl (see Recipes)
2. Defatting medium (see Recipes)
3. Collagenase solution (see Recipes)
4. Expansion medium (see Recipes)
5. Osteogenic differentiation medium (see Recipes)
6. Biochemical stimulation medium (see Recipes)
7. Alizarin Red S solution (see Recipes)
8. Calcein staining solution (see Recipes)
Recipes
1. 1.2 N hydrochloric acid (HCl)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 12.1 N HCl | 1.2 N | 40 mL |
| Deionized (DI) water | n/a | 360 mL |
| Total | 400 mL |
Storage: Room temperature in an acid chemical storage cabinet.
2. Defatting medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Chloroform | 50% | 200 mL |
| Methanol | 50% | 200 mL |
| Total | 400 mL |
Storage: Room temperature in a flammable chemical storage cabinet.
3. Collagenase solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| α-MEM | 1× | 4 mL |
| Collagenase, Type II | 800 U | 40 μL |
| Total | 4.04 mL |
Storage: Collagenase at -20 °C. Once reconstituted at 4 °C, use immediately.
4. Expansion medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| α-MEM powder | 10.1 g/L | 10.1 g |
| NaHCO3 | 2.2 g/L | 2.2 g |
| FBS | 10% | 100 mL |
| Pen/Strep | 1% | 10 mL |
| DI water | n/a | 890 mL |
| Total | 1 L |
Storage: Refrigerator at 4 °C; use within 6 weeks.
5. Osteogenic differentiation medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| α-MEM | 1× | 49.42 mL |
| β-glycerophosphate | 10 mM | 500 μL |
| L-ascorbic acid | 125 μM | 80 μL |
| Total | 50 mL |
Storage: Refrigerator at 4 °C, use within 6 weeks.
6. Biochemical stimulation medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| α-MEM | 1× | 19.96 mL |
| Vitamin D (VD3) | 10 nM | 20 μL |
| Prostaglandin E2 (PGE2) | 1 μM | 20 μL |
| Total | 20 mL |
Storage: Refrigerator at 4 °C, use within 1 week.
7. Alizarin Red S solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Alizarin Red S | 20 mM | 136.9 mg |
| DI water | n/a | 20 mL |
| Total | 20 mL |
Storage: Room temperature or 4 °C, use within 6 months.
8. Calcein staining solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Calcein (from 1 M stock) | 1 μM | 20 μL |
| DI water | n/a | 20 mL |
| Total | 20 mL |
Storage: Room temperature or 4 °C, use within 6 months.
Laboratory supplies
1. Deionized (DI) water (Milli-Q)
2. Scalpel (Graham Field, catalog number: 2975#10)
3. Razor blade (AccuTec Blades, catalog number: AGBL-7033-0000)
4. Biopsy punch 6 mm (Fisher Scientific, catalog number: 12-460-412)
5. Biopsy punch 10 mm (Fisher Scientific, catalog number: NC9226137)
6. 48-well plate (Falcon, catalog number: 353078)
7. 96-well plate (Fisher Scientific, catalog number: FB012931)
8. T-25 flask (Thermo Fisher Scientific, catalog number: 156340)
9. T-75 flask (Fisher Scientific, catalog number: FB012937)
10. T-175 flask (Fisher Scientific, catalog number: NC1932996)
11. 10 cm untreated Petri dish (Fisher Scientific, catalog number: FB0875712)
12. 1.5 mL microtube (Fisher Scientific, catalog number: 05-408-129)
13. 0.6 mL microtube (Fisher Scientific, catalog number: 05-408-128)
14. 50 mL Falcon conical tube (Fisher Scientific, catalog number: 14-432-22)
Supplies for bone demineralization
15. Bovine femurs (local slaughterhouse or grocery store, age 30–42 months according to USDA)
16. 20-inch hand bone saw (KATA SK5)
17. Cyclic hydrostatic pressure chamber (in-house production)
18. Stainless-steel chamber (in-house production)
19. Arduino controller (in-house production)
Equipment
1. Cryostat (Thermo Fisher Scientific, EprediaTM, catalog number: 95-711-0L, model: CryoStar NX70)
2. Centrifuge (Thermo Fisher Scientific, catalog number: 75004221, model: SorvallTM LegendTM X1 Centrifuge)
3. Tissue culture incubator (Thermo Fisher Scientific, catalog number: 51033557, model: HeracellTM VIOS 160i CO2 Incubator)
4. EVOS XL Core Imaging System (Thermo Fisher Scientific, catalog number: AMEX1000)
5. EVOS M7000 imaging system (Invitrogen, catalog number: AMF7000)
6. -80 °C freezer (Thermo Fisher Scientific, catalog number: TSU400D)
7. -20 °C freezer (Thermo Fisher Scientific, catalog number: FBG25FSSA)
8. 4 °C fridge (Thermo Fisher Scientific, catalog number: GTFBG49RPGA)
9. Auto-Fill LN2 Cryo (Thermo Fisher Scientific, catalog number: 7400, model: CryoPlus 1)
10. Radiographic imaging (Faxitron X-Ray LLC, model: MX-20 Cabinet X-ray System)
11. X-ray scanning (Caliper LifeSciences, model: IVIS Spectrum-CT)
12. Second harmonic generation (SHG) multiphoton microscopy (Nikon, model: A1RMP)
13. Microplate reader (Biotek, model: Synergy 2)
14. Inverted fluorescent microscope (Etaluma, model: Lumascope 720)
15. Resonant scanning multiphoton microscope (Nikon, model: A1MP+)
16. Confocal microscope (Carl Zeiss Microscopy, model: Zeiss Cell Observer SD)
17. Box furnace (Thermo Fisher Scientific, catalog number: 10-549-166)
Software and datasets
1. FIJI (ImageJ 1.54)
2. EVOS Fluorescence Microscope Software
3. NIS-Elements Imaging Software
4. Microsoft Excel
5. Adobe Illustrator
6. BioRender (https://www.biorender.com/)
Procedure
文章信息
稿件历史记录
提交日期: Mar 21, 2025
接收日期: May 11, 2025
在线发布日期: May 26, 2025
出版日期: Jun 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Amin, S., Yoon, H., Choi, D., Hsu, Y. and Lee, J. (2025). Murine Osteoblast and Osteoclast Co-culture on Demineralized Bone Paper for Bone Remodeling. Bio-protocol 15(11): e5339. DOI: 10.21769/BioProtoc.5339.
分类
生物工程 > 生物医学工程
细胞生物学 > 细胞分离和培养 > 共培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













