- EN - English
- CN - 中文
Local Iontophoretic Application for Pharmacological Induction of Long-Term Synaptic Depression
局部电泳给药诱导长期突触抑制的方法
(§Technical contact: bolifirov@biph.kiev.ua) 发布: 2025年06月05日第15卷第11期 DOI: 10.21769/BioProtoc.5338 浏览次数: 1880
评审: Anonymous reviewer(s)
Abstract
Long-term depression (LTD), a key form of synaptic plasticity, is typically induced through regulated Ca2+ entry via NMDA receptors and achieved by prolonged (up to hundreds of seconds) low-frequency presynaptic stimulation or bath application of NMDA receptor agonists. Electrophysiological approach to LTD induction requires specialized equipment, while bath applications limit productivity, as only one neuron per sample may be recorded. Here, we present a simple and effective protocol for pharmacological modeling of LTD in primary cultured neurons. This approach relies on highly localized iontophoretic application of NMDA, which induces LTD in individual cells, enhancing experimental throughput. We have analyzed spatio-temporal patterns of iontophoretic drug delivery and demonstrated how this technique may be combined with electrophysiological and live-cell imaging approaches to investigate LTD-related changes in synaptic strength and Ca2+-dependent signaling of neuronal Ca2+ sensor proteins.
Key features
• Easy, fast, and reliable induction of LTD in primary cultured neurons using iontophoretic NMDA application.
• Suitable for the application of any ionic water-soluble compound and compatible with simultaneous multicolor fluorescence imaging and electrophysiological recording.
• This protocol enables pharmacological targeting of individual neurons, substantially increasing experimental throughput.
Keywords: Calcium (钙信号)Graphical overview
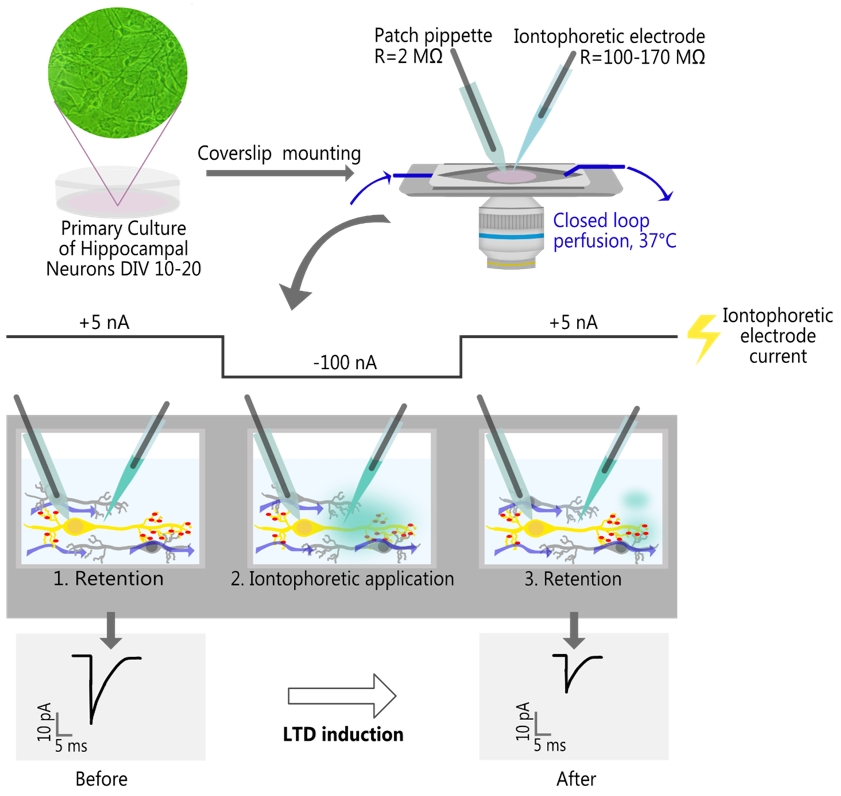
Local iontophoretic application of NMDA, combined with patch-clamp recording of miniature excitatory postsynaptic current (mEPSC) and live-cell fluorescence imaging
Background
Long-term depression (LTD) is an activity-dependent reduction of synaptic strength that is considered to mediate important brain functions such as learning and memory [1–3]. Despite several decades of research, the exact mechanisms of LTD induction and maintenance remain largely elusive. To boost their investigation, easy and reliable methods for eliciting LTD are required. Currently, LTD induction is achieved through either an electrophysiological or pharmacological approach. Both approaches cause a prolonged elevation (lasting up to several hundred seconds) in intracellular free calcium ion concentration ([Ca2+]i), leading to AMPA receptor internalization [3,4]. Electrophysiologically, LTD may be modeled by performing low-frequency stimulation (1 Hz for 200–300 s) of afferent fibers or presynaptic neurons and holding postsynaptic neurons at -40 mV to relieve the Mg2+ block on NMDA receptors (NMDARs) [5–8]. This method requires specialized equipment as well as prior identification of monosynaptically connected neurons, which can be challenging when working with primary cultures. The pharmacological approach to LTD induction is simpler and involves bath application of NMDA (20–50 μM) in the presence of NMDA receptor coactivator glycine (10–20 μM) [6,8–12]. Yet, bath application creates an unpredictable spatio-temporal profile of NMDA concentration and affects all cells in the sample, limiting recordings to a single neuron per sample. To circumvent the drawbacks of previously developed protocols and increase experimental throughput, LTD induction may be accomplished with iontophoresis, a method for rapid and localized drug delivery that is widely used in pharmacological and biophysical studies [13–16]. However, the potential use of iontophoretic application for LTD induction has never been explored.
Here, we provide a detailed protocol of iontophoretic NMDA application for LTD induction in primary cultured hippocampal neurons and describe how it may be coupled with electrophysiological recordings and multicolor epifluorescent imaging. To validate the new protocol, we showed that iontophoretic application of NMDA (100 mM in the electrode) for 1 min produced a long-lasting reduction in the amplitudes of miniature excitatory postsynaptic currents (mEPSCs) and elicited LTD-associated Ca2+-dependent translocation of hippocalcin (HPCA), a neuronal Ca2+ sensor protein, from the cytosol to the neuronal plasma membrane [17,18]. We have also demonstrated that the iontophoresis produced a highly localized concentration gradient, enabling pharmacological targeting of individual neurons. This feature provides a significant advantage over standard bath application, as it allows performing experiments on multiple cells within a single coverslip. This is especially beneficial when working with transfected neurons, given the low success rate of exogenous expression.
It is worth noting that this protocol has great potential for modifications. First, short (up to 10 s) iontophoretic NMDA applications might be used to study various Ca2+-dependent processes. Second, this protocol may be modified to rapidly and locally apply any ionic water-soluble compounds, e.g., glycine or DHPG, to induce long-term potentiation or mGluR-dependent long-term depression of synaptic transmission, respectively [12,19]. Finally, this protocol may be easily combined with advanced microscopy techniques such as confocal [8,12], total internal reflection fluorescence (TIRF) [8–10], and stimulated emission depletion (STED) microscopy [12].
Materials and reagents
Biological materials
1. Primary neuronal culture DIV 10–20 (see [20,21] for hippocampal culture, see [21] for cortical culture)
2. Plasmids for transient transfection: HPCA-EYFP [17], PSD-95-pTagRFP (Addgene #52671)
Note: This protocol does not cover procedures for the cultivation and transient transfection of neurons.
Reagents
1. NaCl (MilliporeSigma, catalog number: S9625)
2. KCl (MilliporeSigma, catalog number: P3911)
3. CaCl2 (MilliporeSigma, catalog number: C3881)
4. MgCl2·6H2O (MilliporeSigma, catalog number: M2670)
5. NaOH (MilliporeSigma, catalog number: S8045)
6. KOH (MilliporeSigma, catalog number: P1767)
7. Methanesulfonic acid (MilliporeSigma, catalog number: 47356)
8. Magnesium ATP (MilliporeSigma, catalog number: A9187)
9. Sodium GTP (MilliporeSigma, catalog number: G8877)
10. Disodium phosphocreatine (MilliporeSigma, catalog number: P7936)
11. EGTA (MilliporeSigma, catalog number: E0396)
12. HEPES (MilliporeSigma, catalog number: H7523)
13. Glucose (MilliporeSigma, catalog number: G7528)
14. Anhydrous DMSO (MilliporeSigma, catalog number: D2650)
15. NMDA (MilliporeSigma, catalog number: M3262)
16. Glycine (MilliporeSigma, catalog number: G7126)
17. Tetrodotoxin citrate (TTX) (Tocris, catalog number: 1069); prepare 5 mM stock solution according to manufacturer protocol
18. Alexa Fluor 594 hydrazide (Thermo Fisher, catalog number: A10438)
19. Fluo-4/AM (Thermo Fisher, catalog number: F14201)
20. Ethanol, 96% v/v
Solutions
1. HEPES buffered saline (HBS) with 1 mM/0.3 mM Mg2+ (see Recipes)
2. KCl-methanesulfonate intracellular solution (see Recipes)
3. HEPES (0.5 M, pH 7.3, stock solution in ddH2O) (see Recipes)
4. NMDA (100 mM, stock solution in 10 mM HEPES, pH 7.3) (see Recipes)
5. Glycine (100 mM, stock solution in 10 mM HEPES, pH 7.3) (see Recipes)
6. Alexa Fluor 594 (5 mM, stock solution in 10 mM HEPES, pH 7.3) (see Recipes)
7. Fluo-4/AM (5 mM, stock solution in DMSO) (see Recipes)
Recipes
A. Extracellular solution
Note: Alternative compositions for an extracellular solution only differ by the concentration of Mg2+, which is crucial for studying NMDAR-dependent signaling, so you could choose only one that is appropriate for the particular experimental design.
1. HEPES buffered saline (HBS) with 1 mM/0.3 mM Mg2+
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaCl | 150 mM | 4,383 mg |
| KCl | 2.5 mM | 92.5 mg |
| CaCl2 | 2 mM | 147 mg |
| MgCl2·6H2O | 1 mM/0.3 mM | 102 mg/30.5 mg |
| HEPES | 10 mM | 1,192 mg |
| ddH2O | n/a | 500 mL |
I = 160 mM, osmolarity ~290–310 mOsm/L.
Notes:
1. Dissolve all components in ~3/4 of the final volume, adjust pH to 7.3 with 2–3 M NaOH solution, and adjust the volume to the final value. Filter and store at 4 °C for no longer than 6 months.
2. Add glucose before the start of the experiment (9 mg per 10 mL of HBS).
B. Intracellular solution
2. KCl-methanesulfonate intracellular solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| KCl | 10 mM | 18.64 mg |
| Methanesulfonic acid | 135 mM | 324.37 mg |
| Magnesium ATP | 4 mM | 50.7 mg |
| Sodium GTP | 0.4 mM | 5.23 mg |
| Disodium phosphocreatine | 5 mM | 31.89 mg |
| EGTA | 0.5 mM | 4.75 mg |
| HEPES | 10 mM | 59.5 mg |
| ddH2O | n/a | 25 mL |
Osmolarity ~270–290 mOsm/L.
Note: Methanesulfonic acid is highly viscous; handle it carefully. The necessary volume is 219 μL (ρ = 1.481 g/mL). Dissolve all components in ~3/4 of the final volume, adjust pH to 7.3 with KOH, and adjust the volume to the final value. Make 0.5 mL aliquots and store at -20 °C for no longer than 12 months.
C. Pharmacological compounds and dyes
3. HEPES (0.5 M, pH 7.3, stock solution in ddH2O)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HEPES | 0.5 M | 2.979 mg |
| ddH2O | n/a | 25 mL |
Note: Dissolve all components in ~3/4 of the final volume, adjust pH to 7.3 with 2–3 M NaOH solution, and adjust the volume to the final value. Filter and store at 4 °C. This stock buffer solution is used to dissolve pharmacological compounds and dyes.
4. NMDA (100 mM, stock solution in 10 mM HEPES, pH 7.3)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NMDA | 100 mM | 7.36 mg |
| HEPES stock solution (Recipe 3) | 10 mM | 10 μL |
| ddH2O | n/a | 490 μL |
Note: Given that it may be difficult to weigh small quantities of NMDA, weigh the compound directly in the 1.5 mL reaction tube, and use the following relationship to estimate the appropriate volume of stock solution components: 66.608 μL of ddH2O and 1.359 μL of 0.5 M HEPES, pH 7.3 per1 mgof NMDA (MW = 147.13 g/mol). Add ddH2O first, then the buffer stock solution; dissolve the compound with active tube agitation or vortexing. Briefly centrifuge the tube to collect all the solution at the bottom, aliquot it, and store at -20 °C for no longer than 6 months.
5. Glycine (100 mM, stock solution in 10 mM HEPES, pH 7.3)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glycine | 100 mM | 37.55 mg |
| HEPES stock solution (Recipe 3) | 10 mM | 0.1 mL |
| ddH2O | n/a | 4.9 mL |
Note: Add ddH2O first, then the buffer stock solution; dissolve the compound, aliquot it, and store at -20 °C for no longer than 10–12 months.
6. Alexa Fluor 594 (5 mM, stock solution in 10 mM HEPES, pH 7.3)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Alexa Fluor 594 | 5 mM | 1 mg |
| HEPES stock solution (Recipe 3) | 10 mM | 5.27 μL |
| ddH2O | n/a | 258.31 μL |
Note: Volume estimated for 1 mg of dye (MW = 758.79 g/mol); add ddH2O first, then the buffer stock solution, and dissolve the compound by shaking or vortexing. Briefly centrifuge the tube to collect all the solution at the bottom, aliquot it, and store at -20 °C no longer than 10–12 months.
7. Fluo-4/AM (5 mM, stock solution in DMSO)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Fluo-4/AM | 5 mM | 50 μg |
| DMSO | n/a | 9.12 μL |
Note: Volume estimated for 50 μg of dye (MW = 1096.95 g/mol); add DMSO and dissolve the dye with gentle pipetting; use only anhydrous DMSO; the presence of H2O in the solvent may dramatically affect dye solubility. Briefly centrifuge the tube, aliquot the solution, and store at -20 °C for no longer than 10–12 months. Try to reduce the number of freeze-thaw cycles of aliquots and check for the presence of precipitate before using. Pipette the solution if necessary.
Laboratory supplies
1. 35 mm Petri dish (CellStar, catalog number: 627160)
2. 1.5 mL reaction tube (Greiner, catalog number 616 201)
3. Glass capillaries with filament O.D. 1.5 mm, I.D. 0.86 mm (Sutter Instrument, catalog number: BF150-86-10)
4. 2.5 mm box-shaped heating filament for microelectrode puller (Sutter Instruments, catalog number: FB255B)
5. Tygon® tube O.D. 2.4 mm I.D. 0.7 mm (Saint-Gobain, catalog number: B-44-4X)
6. Silicon tubing (VWR, catalog number: 228-0701)
7. Luer to tube assortment kit (WPI, catalog number: 504954)
8. Luer valve assortment kit (WPI, catalog number: 14011)
9. Luer-lock syringes (any available with a volume of 1, 10, and 20 mL)
10. Silicone grease (Warner Instruments, catalog number: W4 64-0275)
11. Cross-locking tweezer (Dumont, catalog number: 0202-N5-PS)
12. MicroFil flexible needle (WPI, catalog number: MF28G67-5)
13. Alcohol burner
Equipment
A. Imaging and electrophysiology
1. Inverted microscope (Olympus, model: IX71)
2. 40× oil-immersion high-aperture objective (Olympus, model: UApo/340 40×/1.35)
3. Optical filters set (suitable for selected fluorophores, in our case, Chroma, model: 69008)
4. Dual-view splitter (Optical Insights, model: MSMI-DV-FC)
5. Digital camera (PCO, model: SensiCam QE)
6. Vibration isolation table (CleanBench, model: TMC)
7. Imaging control unit (TILL Photonics, model: ICU2)
8. Monochromator (TILL Photonics, model: Polychrome V)
9. Patch clamp amplifier (HEKA, model: EPC 10)
10. Micromanipulators (Sutter Instrument, model: MPC-200)
11. Ag/AgCl pellets (WPI, catalog number: EP1)
12. Experimental chamber (Warner Instruments, model: RC-25 or DIY analog)
13. Microelectrode puller (Sutter Instrument, model: P-97)
14. BNC cables
15. Computer for setup control
16. Compact benchtop incubator (any capable of maintaining 37 °C, e.g., VWR, model: INCU-Line IL 10)
B. Gravity-fed perfusion system (optional)
17. Peristaltic pump (Kamoer, model: DIPump550)
18. Flow dial (Bioscience Tools, model: PS-FLOW)
19. Temperature controller (Warner Instruments, model: TC-344B)
20. In-line heater (Warner Instruments, model: SH-27B)
21. Magnetic clamp for outlet tube (Warner Instruments, model: MAG-2)
22. Valve controller (Bioscience Tools, model: cValve-6)
23. Solenoid valves (NResearch Inc., models: 161P021, 161P091)
Note: We validate our protocol in the presence and absence of continuous perfusion, but a closed-loop perfusion system is highly recommended for consistent washout kinetics and reproducibility. See the detailed description of the perfusion system in supplementary Figure S1 and Figure S2; 3D models of perfusion system parts for fused deposition modeling (FDM) printing can be provided upon request.
Software and datasets
A. Experimental setup control
1. PatchMaster (v2x69, HEKA, Germany)
2. Live Acquisition (v2.6.0.12, FEI, Germany)
B. mEPSC analysis
3. Clampfit (v11.3, Molecular Devices, USA)
4. NeuroExpress (v24.8.02, GitHub: github.com/attilaszuc/NeuroExpress-patch-clamp-analysis)
C. Image analysis
5. napari (v0.4.19) [22]
6. domb-napari plugin (v0.3.0) [23]
Procedure
文章信息
稿件历史记录
提交日期: Mar 31, 2025
接收日期: May 6, 2025
在线发布日期: May 27, 2025
出版日期: Jun 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Olifirov, B., Fedchenko, O., Dovgan, A., Babets, D., Krotov, V., Cherkas, V. and Belan, P. (2025). Local Iontophoretic Application for Pharmacological Induction of Long-Term Synaptic Depression. Bio-protocol 15(11): e5338. DOI: 10.21769/BioProtoc.5338.
分类
神经科学 > 细胞机理 > 突触生理学
细胞生物学 > 细胞信号传导 > 突触传递
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link