- EN - English
- CN - 中文
Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
使用康可藻红素刺激冷冻保存的猪外周单个核细胞进行增殖检测,并结合FCS ExpressTM 7.18软件分析
(§Technical contact: mbravo@iastate.edu) 发布: 2025年06月05日第15卷第11期 DOI: 10.21769/BioProtoc.5332 浏览次数: 2622
评审: Shaobin ShangAnonymous reviewer(s)

相关实验方案

外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1449 阅读
Abstract
In vitro lymphocyte proliferation assays are essential for assessing immune responses and antiproliferative drug efficacy. Such assays rely on antigen presentation or mitogen stimulation, with performance determined by reagent concentration and incubation time. Although splenocytes are often used, peripheral blood mononuclear cells (PBMCs) offer more accessible and practical sampling. However, a streamlined protocol for porcine PBMCs proliferation with robust batch analysis has been lacking. We therefore developed a detailed workflow for inducing proliferation in cryopreserved porcine PBMCs using 5 μg/mL concanavalin A (ConA). The protocol covers cell isolation, cryopreservation, ConA stimulation, CD4+ T-cell staining, flow cytometry acquisition and gating on an Attune NxT instrument, and batch analysis with FCS ExpressTM 7.18. This approach yielded 78.9% viable cells, of which 33.8% were CD4+ lymphocytes. Moreover, 93.9% (n = 216) of cells proliferated, yielding up to nine cell generations. Batch analysis in FCS ExpressTM enhanced the accuracy and interpretation of proliferation metrics. This validated protocol provides a reliable framework for generating consistent proliferation data in porcine immunology studies.
Key features
• Optimized proliferation assay for cryopreserved porcine PBMCs: Ideal for immunological studies and biomedical research using swine models.
• Precise acquisition and gating with Attune NxT flow cytometer: Ensures accurate identification and analysis of CD4+ T lymphocytes.
• Robust data analysis using FCS ExpressTM 7.18 software: Facilitates reliable interpretation of lymphocyte proliferation and division indexes.
• Efficient 4.5-day protocol: Enables timely and reproducible experiments in clinical and research settings involving porcine immune cells.
Keywords: Proliferation assay (增殖检测)Graphical overview
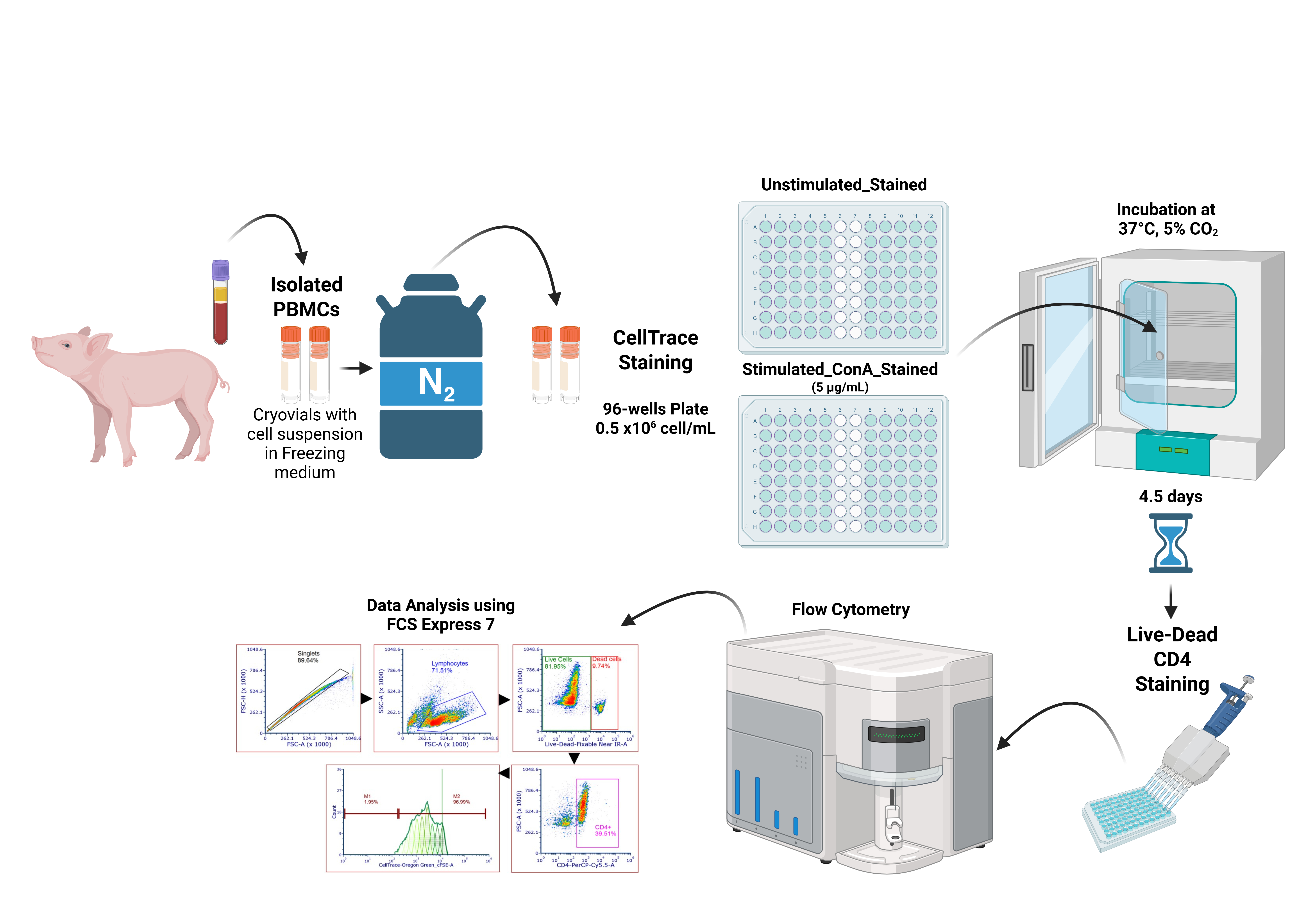
Proliferation assay process from cryopreserved porcine peripheral blood mononuclear cells (PBMCs)
Background
Proliferation assays are fundamental for evaluating both the cytotoxic effects of antiproliferative agents [1,2] and antigen-driven immune responses [3]. These assays rely on either antigen presentation or mitogen stimulation, with concanavalin A (ConA) and phytohemagglutinin (PHA), Ca2+/Mn2+-dependent lectins that bind mannose and glucose residues, serving as indispensable polyclonal stimulants [4,5]. A variety of proliferation readouts have been described: thymidine‐analog incorporation like BrdU [6,7], generational‐tracking dyes such as CFSE and CellTrace [8–10], and detection of proliferation‐associated antigens (Ki67, PCNA) by flow cytometry or western blot [11,12]. Data analysis platforms, including ModFit, FlowJo, and FCS ExpressTM, facilitate automated calculation of division and proliferation indexes [13–16].
In swine immunology, proliferation assays underpin vaccine evaluation, disease pathogenesis research, and immune surveillance. However, porcine peripheral blood mononuclear cells (PBMCs) present unique challenges: cryopreservation can reduce viability by 10%–20% and disproportionately impair CD4 T‐cell recovery, while baseline frequencies of CD4+ subsets vary widely between animals [17,18]. Published protocols (often developed for fresh splenocytes) lack harmonized freezing/thawing procedures, standardized staining protocols, and data analysis workflows, leading to inter‐laboratory variability.
To overcome these limitations, we developed a fully standardized protocol for inducing proliferation in cryopreserved porcine PBMCs. Cells were stimulated with 5 μg/mL ConA, divisions were tracked via CellTraceTM Oregon Green dilution, and gating was performed automatically on an Attune NxT flow cytometer. Data were exported in batch mode and analyzed in FCS ExpressTM 7.18 using fixed‐parameter proliferation fits with a uniform coefficient of variation, fixed peak ratios, and controlled background noise. This workflow yields highly reproducible division and proliferation indexes across large cohorts, outperforming methods that rely on fresh cells or manual gating and providing a robust foundation for porcine immunology studies.
This protocol is broadly applicable in swine disease research as a reliable positive-control assay for benchmarking proliferation. It facilitates optimization of antigen doses, such as microbial proteins or vaccine candidates, by enabling comparative analysis of proliferative responses across titration series, and supports evaluation of immunomodulatory or antiproliferative compounds under real-world cryopreservation conditions. By employing uniform gating and batch-mode analysis, the method also standardizes comparisons among lymphocyte subsets (e.g., CD4+, CD8+, γδ T cells), minimizing inter-assay variability. Moreover, its high-throughput format and consistent readouts make it suitable for large-scale vaccine efficacy and challenge studies, where correlating proliferation metrics with clinical outcomes is critical. Incorporating detailed guidelines for sample handling, staining, acquisition, and batch-mode data analysis, this protocol enhances reproducibility and comparability across cohorts and laboratories, thereby advancing swine immunology and vaccine development efforts.
Although ConA is used here as a nonspecific positive control, this workflow can be directly adapted for antigen-specific proliferation assays. By substituting ConA with the antigen or peptide pool of interest (at a concentration determined by preliminary titration) and including unstimulated and ConA-stimulated wells as negative and maximal controls, respectively, one can reliably quantify antigen-driven CD4+ T-cell proliferation using the same CellTraceTM labeling, flow cytometry acquisition, and batch-mode FCS ExpressTM 7.18 analysis. In a pilot study, we applied this protocol with influenza virus (IAV) nucleoprotein (NP) at 10 μg/mL in pigs with prior exposure and detected antigen-specific CD4+ proliferation, demonstrating the method’s adaptability (data not shown).
Materials and reagents
Biological materials
1. Frozen porcine peripheral blood mononuclear cells PBMCs (5–10 × 106 cells/mL) in freezing media (see Recipes); preserved in liquid nitrogen
Reagents
1. Ficoll-Paque (Cytiva, catalog number: 17-1440-03)
2. Hank’s balanced salt solution (HBSS) (Thermo Fisher Scientific, Gibco®, catalog number: 14715095)
3. 10× red blood cell (RBC) lysis buffer (Santa Cruz Biotechnology, catalog number: sc-296258)
4. Trypan blue 0.4% (Thermo Fisher Scientific, Gibco®, catalog number: 15250061)
5. 1× phosphate buffered saline (PBS) (Thermo Fisher Scientific, Gibco®, catalog number: 10010023)
6. Fetal bovine serum (FBS) (Atlas Biologicals, EqualFetal, catalog number: EF-0500-A); inactivated at 56 °C for 30 min and stored at -20 °C
7. Roswell Park Memorial Institute (RPMI) 1640 buffer, medium without L-glutamine and phenol red-free (Thermo Fisher Scientific, Gibco®, catalog number: 32404014); stored at 4 °C
8. Penicillin streptomycin: 10,000 U/mL penicillin and 10,000 µg/mL streptomycin (pen-strep) (Thermo Fisher Scientific, Gibco®, catalog number: 15140122); stored at -20 °C
9. 100× GlutaMAX (Thermo Fisher Scientific, Gibco®, catalog number: 35050061)
10. 100× minimum essential medium non-essential amino acids (MEM NEAA) (Thermo Fisher Scientific, Gibco®, catalog number: 11140050); stored at 4 °C
11. 1 M HEPES (Thermo Fisher Scientific, Gibco®, catalog number: 15630080); stored at 4 °C
12. Cytofix/Cytoperm fixation and permeabilization solution (BD Biosciences, catalog number: BDB554722)
13. LIVE/DEAD Fixable Near-IR Dead Cell Stain kit (Thermo Fisher Scientific, Invitrogen, catalog number: L34976); stored at -20 °C
14. CellTraceTM Oregon Green 488 carboxylic acid diacetate, succinimidyl ester (carboxy-DFFDA, SE), cell-permeant, mixed isomers (Thermo Fisher Scientific, Invitrogen, catalog number: C34555); stored at -20 °C
15. PerCP-Cy5.5 mouse anti-pig CD4a, clone 74-12-4, isotype: mouse BALB/c IgG2b, κ (BD, catalog number: 561474); stored at 4 °C
16. UltraComp eBeads Plus compensation beads, stored at 4 °C (Thermo Fisher Scientific, Invitrogen, catalog number: 01-3333-42)
17. ArC reactive beads (Thermo Fisher Scientific, Invitrogen, catalog number: A10346); stored at 4 °C
18. Dimethyl sulfoxide (DMSO) (Millipore-Sigma, catalog number: D2650)
19. Concanavalin A (Cayman Chemical, catalog number: 14951)
20. Isopropanol (VWR Chemicals BDH, CAS number: 67-63-0)
Solutions
1. HBSS 2% FBS (see Recipes)
2. PBMC medium (see Recipes)
3. 1× RBC lysis buffer (see Recipes)
4. Freezing medium (see Recipes)
5. 0.5% PBS/FBS (see Recipes)
6. FACS buffer (see Recipes)
7. 0.008% trypan blue solution (see Recipes)
8. CellTraceTM Oregon Green 488 Carboxy-DFFDA, SE stock solution (see Recipes)
9. CellTraceTM Oregon Green 488 Carboxy-DFFDA, SE working solution (see Recipes)
10. LIVE/DEAD Fixable Near-IR Dead Cell Stain stock solution (see Recipes)
11. LIVE/DEAD Fixable Near-IR Dead Cell Stain working solution (see Recipes)
12. PerCP-CyTM 5.5 mouse anti-pig CD4a working solution (see Recipes)
13. ConA stock solution (see Recipes)
14. ConA working solution (see Recipes)
Recipes
1. HBSS 2% FBS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| HBSS | n/a | 98% |
| FBS | n/a | 2% |
2. PBMC medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI 1640 | n/a | 88% |
| FBS | n/a | 8% |
| Pen-strep | n/a | 1% |
| MEM NEAA | n/a | 1% |
| HEPES | n/a | 1% |
| GlutaMAX | n/a | 1% |
Note: Filter the buffer through a 0.2 μm filter and store it at 4 °C. Use within one month.
3. 1× RBC lysis buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 10× RBC lysis buffer | 10× | 10% |
| Purified Millipore water | n/a | 90% |
Note: Filter the buffer through a 0.2 μm filter and store it at 4 °C.
4. Freezing medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PBMC medium (Recipe 2) | n/a | 60% |
| FBS | n/a | 30% |
| DMSO | n/a | 10% |
Note: Filter the buffer through a 0.2 μm filter and store it at 4 °C.
5. 0.5% PBS/FBS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PBMC medium (Recipe 2) | n/a | 99.5% |
| FBS | n/a | 0.5% |
Note: Filter the buffer through a 0.2 μm filter and store it at 4 °C.
6. FACS buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1× PBS | n/a | 99% |
| FBS | n/a | 1% |
Note: Filter the buffer through a 0.2 μm filter and store it at 4 °C.
7. 0.008% trypan blue solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1× PBS | n/a | 9.8 mL |
| 0.4% trypan blue | n/a | 0.2 mL |
| Total | n/a | 10 mL |
8. CellTraceTM Oregon Green 488 Carboxy-DFFDA, SE stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CellTrace-Oregon Green 488 Carboxy-DFFDA | n/a | 1 vial |
| DMSO | n/a | 50 μL |
Note: Prepare the stock solution before use (1 h) because the DMSO from the kit is frozen, and you need to wait until it thaws. Keep it at room temperature before using it.
9. CellTraceTM Oregon Green 488 Carboxy-DFFDA, SE working solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CellTraceTM Oregon Green 488 Carboxy-DFFDA, SE stock solution (Recipe 8) | n/a | 0.3 μL |
| 1× PBS | n/a | 1 mL |
Note: Prepare the working solution immediately before use (within 5–10 min). Keep it on ice and protected from light to preserve reagent activity.
10. LIVE/DEAD Fixable Near-IR Dead Cell Stain stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| LIVE/DEAD Fixable Near-IR Dead Cell Stain | n/a | 1 vial |
| DMSO | n/a | 50 μL |
Note: Prepare the stock solution before use (1 h) because the DMSO from the kit is frozen, and you need to wait until it thaws. Keep it at room temperature and covered from light before using it.
11. LIVE/DEAD Fixable Near-IR Dead Cell Stain working solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| LIVE/DEAD Fixable Near-IR Dead Cell Stain stock solution (Recipe 10) | n/a | 1 μL |
| 1× PBS | n/a | 99 μL |
Note: Prepare the working solution before use (1 h). Keep it on ice, covered from light.
12. PerCP-CyTM 5.5 mouse anti-pig CD4a working solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PerCP-CyTM 5.5 mouse anti-pig CD4a (200 μg/mL) | 2 μg/mL | 1 μL |
| FACS buffer (Recipe 6) | n/a | 99 μL |
Note: Keep it on ice and covered from light.
13. ConA stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| ConA, lyophilized powder | 1 mg/mL | 1 mg |
| 1× PBS | n/a | 1 mL |
Note: Keep it on ice before use.
14. ConA working solution (for 50 wells of 150 μL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| ConA stock solution (1 mg/mL) (Recipe 13) | 5 μg/mL | 37.5 μL |
| PBMC medium (Recipe 2) | n/a | 7462.5 μL |
Note: Prewarm the ConA working solution before adding it to the cells.
Laboratory supplies
1. 15 mL SepMate tubes (STEMCELL Technologies, catalog number: 85415)
2. 15 mL conical centrifuge tubes NUNC (Thermo Fisher Scientific, NuncTM, catalog number: 12-565-269)
3. 10 mL serological pipettes (Thermo Fisher Scientific, Fisherbrand, catalog number: 13-678-11E)
4. 25 mL serological pipettes (Thermo Fisher Scientific, Fisherbrand, catalog number: 13-678-11)
5. Sterile pipette tips 250 μL filtered, non-autoclavable, sterile, DNase- and RNase-free (Biotix, catalog number: M-0250-9NC)
6. Sterile pipette tips 1000 μL, non-autoclavable, sterile, DNase- and RNase-free (Thermo Fisher Scientific, Fisherbrand, catalog number: 02-707-404)
7. DNA LoBind 1.5 mL tubes (Eppendorf, catalog number: 022431021)
8. 500 mL bottles (Thermo Fisher Scientific, Nalgene, catalog number: 02540263)
9. Filter unit 1 L (Thermo Fisher Scientific, Nalgene, catalog number: 0974103)
10. 2 mL cryovials (Corning, catalog number: 430488)
11. Freezing container (Thermo Fisher Scientific, catalog number: 15-350-50)
12. Cell culture microplate, 96 well, PS, U-bottom, clear (Greiner Bio-One, catalog number: 650-180)
13. 40 μm Flowmi cell strainer (SP Bel-Art, catalog number: H13680-0040)
14. Hemocytometer counting chamber (Hausser Scientific, catalog number: 3200)
15. Reagent reservoirs 25 mL (VWR, catalog number: 89094-662)
Equipment
1. Laminar flow hood, Class II Type A2 (Nuaire Biological Safety Cabinets)
2. Incubator, 37 °C, 5% CO2 (Caron Oasis CO2)
3. Inverted microscope (Olympus, model: CKX41)
4. Sorvall Legend RT+ centrifuge (Thermo Fisher Scientific, catalog number: 75004377, model: D-37520 Osterode)
5. Attune NxT Gen, Acoustic Focusing Cytometer with Autosampler (Thermo Fisher Scientific, InvitrogenTM, model: AFC2)
6. Multichannel pipette 30–300 µL (Fisherbrand Elite, catalog number: OU38517)
Software and datasets
1. Attune NxT software v3.2.1 (Thermo Fisher Scientific, InvitrogenTM)
2. FCS ExpressTM 7.18 software, Research Edition (De Novo Software 2023)
3. GraphPad Prism 10 (Dotmatics)
Procedure
文章信息
稿件历史记录
提交日期: Jan 26, 2025
接收日期: May 6, 2025
在线发布日期: May 26, 2025
出版日期: Jun 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bravo-Parra, M., Nelli, R. K., Yen, L., Castillo, G., Mora-Díaz, J. C., Castillo-Espinoza, A. F. and Giménez-Lirola, L. G. (2025). Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software. Bio-protocol 15(11): e5332. DOI: 10.21769/BioProtoc.5332.
分类
免疫学 > 免疫细胞功能 > 综合
免疫学 > 免疫细胞分离 > 淋巴细胞
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











