- EN - English
- CN - 中文
An Automated Imaging Method for Quantification of Changes to the Endomembrane System in Mammalian Spheroid Models
基于哺乳动物类器官球模型的内膜系统变化自动成像定量方法
(*Contributed equally to this work, §Technical contact: margaritha.mysior@ucd.ie) 发布: 2025年06月05日第15卷第11期 DOI: 10.21769/BioProtoc.5331 浏览次数: 1660
评审: Dogan Can KirmanAndrea GramaticaAnonymous reviewer(s)
Abstract
Three-dimensional cell models, such as spheroids, represent a more physiological arrangement in which cells can grow, allowing them to develop cell–cell interactions in all dimensions. The most common methods for growing spheroids are scaffold-based, typically using either extracellular matrix or hydrogels as a physical support for the cellular assembly. One key problem with this approach is that the spheroids that are produced can be highly variable in size and shape. The protocol presented here allows for the systematic production of uniform spheroids in a short time frame by utilising a micropatterned plate. We show that spheroids can be used to investigate fundamental research questions, such as how the endomembrane system is organised in cells. Our protocol can be used in a manual or automated manner, potentially allowing scaling up for screening applications. Furthermore, without the complication of removing the spheroids from the extracellular matrix or hydrogel, as would be required in scaffold-based systems, spheroids can easily be used in other downstream applications.
Key features
• This work builds upon the method developed by Monjaret et al. [1] and establishes a robust method to produce spheroids from HeLa Kyoto cells.
• This protocol generates consistent populations of spheroids that can be used to investigate organelle biology and membrane trafficking pathways.
• Entire spheroids can be analysed in a volumetric manner.
• This method can be used in both manual and automated pipelines, thereby facilitating use in high-throughput and high-content screens.
Keywords: 3D cell culture (三维细胞培养)Graphical overview
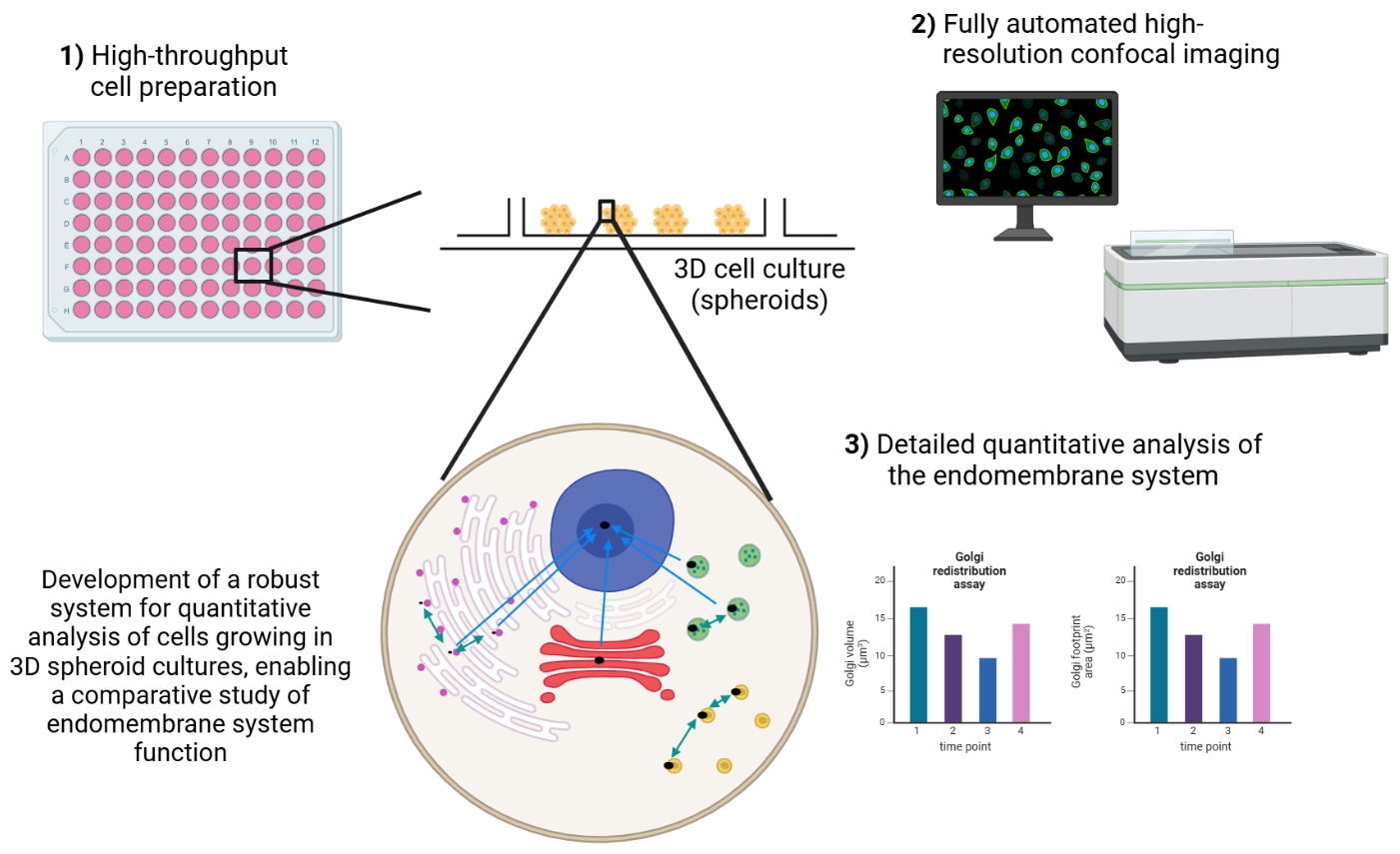
Background
Three-dimensional (3D) cell models are increasingly utilised in research as they more closely mimic the arrangement of cells in the human body when compared to classical monolayer cultures [2–4]. 3D cell models can be broadly classified into two categories: spheroids and organoids. Organoids are organ-like cell assemblies mainly grown from stem cells [2,4–6]. The primary technique used to grow organoids is an extracellular matrix material with the cells embedded in the matrix, the molecules of which mimic the in vivo environment [7–10]. Spheroids can be grown from established cell lines and stem cells. If cancer cell lines are used, they are termed multicellular tumour spheroids [3,11]. There are many different techniques for growing spheroids. These techniques can be classified into scaffold-based and scaffold-free methods, with each having its particular advantages and disadvantages [12–16]. Many of these methods produce spheroids that are heterogeneous in both size and shape, making comparisons across any population challenging. Scaffold-free methods most commonly utilise round-bottom ultra-low attachment plates for spheroid growth, which, although allowing high uniformity of spheroid size, suffer from the key disadvantage of only producing a single spheroid per well. This therefore precludes their use in large-scale screening studies. The most suitable method for growing spheroids also depends on the cell lines and types needed, in addition to the specific research question being addressed.
3D cell models have a wide number of applications, including in cancer research, developmental cell biology, toxicology studies, and drug development [12,14,15,17–19]. However, to date, fundamental research areas have largely underutilised them [16] despite their potential being proposed almost twenty years ago [20,21]. The protocol that we have developed allows the routine production and use of uniform-sized spheroids in fundamental and applied research studies. This has the potential to significantly simplify the use of spheroids in many important applications, such as toxicity studies. These experiments are often carried out by growing spheroids in basement membrane extracts, which, although having the advantage of being relatively technically simple, results in an extremely diverse population of spheroids being produced within a single well [18]. Importantly, the size of spheroids produced allows the investigation of cellular behaviour at a subcellular scale, and the entire spheroid can be fully imaged with confocal microscopy, allowing them to be used for volumetric image analysis measurements. Together, this means that information can be extracted at subcellular, cellular, and whole-spheroid levels from the same sample. Furthermore, our method produces several hundred spheroids per well in a multi-well plate, thereby enabling their easy use in downstream applications, such as RNA extraction and qPCR.
Materials and reagents
Biological materials
1. HeLa Kyoto cells (human cervical cancer cell line, RRID; CVCL-1922)
2. HeLa Kyoto cells stably expressing EGFP (generated in-house [22])
3. HeLa Kyoto cells stably expressing EGFP-Rab5A (generated in-house [22])
4. HeLa Kyoto cells stably expressing EGFP-Rab6A (generated in-house [22])
Reagents
1. Dulbecco’s modified Eagle medium (DMEM) with L-Glutamine, pyruvate, 1 g/L glucose (Life Technologies Europe BV, Gibco, catalog number: 31885023); store at 4 °C
2. Value heat-inactivated (HI) fetal bovine serum (FBS) (Life Technologies Europe BV, Gibco, catalog number: A5256801); store at -20 °C
3. L-Glutamine solution (200 mM) (Life Technologies Europe BV, Gibco, catalog number: 25030024); store at -20 °C
4. 0.05% Trypsin-EDTA (1×), phenol red (Life Technologies Europe BV, Gibco, catalog number: 25300054); store at -20 °C
5. G418 sulfate (50 mg/mL) (Fisher Scientific, Corning, catalog number: 30-234-CI); store at 4 °C
6. PBS tablets (Fisher Scientific, catalog number: 12821680); store at room temperature (RT)
7. Dimethyl sulfoxide (DMSO) (VWR, catalog number: A3672.0100); store at RT
8. Glycine (Fisher BioReagents, catalog number: BP3811); store at RT
9. Bisbenzimide H 33342 trihydrochloride (Hoechst 33342) (Merck, catalog number: 14533); store at 4 °C
10. Triton X-100 (Merck, catalog number: T9284-500ml); store at RT
11. Rabbit anti-GM130 (clone: D6B1) (Cell Signalling Technologies, catalog number: 12480); store at -20 °C
12. Mouse anti-EEA1 (clone: 14/EEA1) (BD Biosciences, catalog number: 610457); store at -20 °C
13. Mouse anti-LAMP1 (clone: H4A3) (Developmental Studies Hybridoma Bank, catalog number: H4A3, supernatant); store at 4 °C
14. Alexa Fluor 568 goat anti-rabbit IgG (H+L) highly cross-adsorbed (Thermo Fisher, catalog number: A11036); store at -20 °C
15. Alexa Fluor 647 goat anti-mouse IgG (H+L) highly cross-adsorbed (Thermo Fisher, catalog number: A21236); store at -20 °C
16. Sodium azide (Merck, catalog number: S2002-25g); store in a well-ventilated place
Caution: Sodium azide is toxic.
17. Brefeldin A (BFA) (Merck, catalog number: B7651); store at 4 °C
18. Nocodazole (Merck, catalog number: M1404); store at 4 °C
19. Deionised water (dH2O)
20. Paraformaldehyde (PFA) (Merck, catalog number: P6148), store at 4 °C
Caution: PFA is toxic.
21. Calcium chloride dihydrate (Merck, catalog number: C3306)
22. Magnesium chloride hexahydrate (Fisher Scientific, catalog number: M/0600/53)
Solutions
1. Complete medium (see Recipes)
2. Complete medium with G418 (see Recipes)
3. 1× PBS (see Recipes)
4. Hoechst 33342 solution (see Recipes)
5. 1 M glycine solution (see Recipes)
6. 30 mM glycine solution (see Recipes)
7. 10% Triton X-100 solution (see Recipes)
8. 0.5% Triton X-100 solution (see Recipes)
9. 10% sodium azide solution (see Recipes)
10. BFA solution (see Recipes)
11. Nocodazole solution (see Recipes)
12. 1 M calcium chloride (see Recipes)
13. 1 M magnesium chloride (see Recipes)
14. 3% PFA (see Recipes)
Recipes
1. Complete medium
Thaw the HI FBS and L-Glutamine at 37 °C for 1 h or at 4 °C overnight. Remove 55 mL from a fresh bottle of DMEM and put it into two 50 mL conical tubes. Mix HI FBS and L-Glutamine with DMEM. Store at 4 °C. All steps are carried out in a Class II biological safety cabinet.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM 1 g/L glucose | 445 mL | |
| HI FBS | 10% | 50 mL |
| L-Glutamine | 1% | 5 mL |
| Total | 500 mL |
2. Complete medium with G418
Follow the same steps as in Recipe 1; remove 62 mL from a fresh bottle of DMEM. Additionally, add the G418. Store at 4 °C. All steps are carried out in a Class II biological safety cabinet.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM 1 g/L glucose | 438 mL | |
| HI FBS | 10% | 50 mL |
| L-Glutamine | 1% | 5 mL |
| G418 | 700 μg/mL | 7 mL |
| Total | 500 mL |
3. 1× PBS
Dissolve one tablet in 100 mL of dH2O. Prepare one bottle for cell culture and one bottle for main lab work. The 1× PBS that is used in cell culture should be autoclaved; 1× PBS for main lab work does not necessarily need to be autoclaved. Store at RT.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PBS tablet | 1× | 1 tablet |
| dH2O | n/a | 100 mL |
| Total | 100 mL |
4. Hoechst 33342 solution
Weigh 1 mg of Hoechst 33342 and put it into a tube with 1 mL of dH2O. Vortex the tube. Once the Hoechst 33342 has dissolved, centrifuge at 140× g for 1 min. Prepare 50 μL aliquots and store at -20 °C. These are stable for at least one year. Protect the aliquots from light when in use.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Hoechst 33342 | 1 mg/mL | 1 mg |
| dH2O | n/a | 1 mL |
| Total | 1 mL |
5. 1 M glycine solution
Weigh the glycine and put it into a 50 mL conical tube. Add the PBS (not necessarily autoclaved) and vortex the conical tube to dissolve the glycine. Store at 4 °C.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glycine | 1 M | 3.753 g |
| PBS (Recipe 3) | n/a | 50 mL |
| Total | 50 mL |
6. 30 mM glycine solution
Add 1.5 mL of 1 M glycine into 48.5 mL of PBS (not necessarily autoclaved) in a 50 mL conical tube. Vortex the conical tube to create a homogenous solution. Store at 4 °C.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M glycine (Recipe 5) | 30 mM | 1.5 mL |
| PBS (Recipe 3) | n/a | 48.5 mL |
| Total | 50 mL |
7. 10% Triton X-100 solution
Slowly aspirate the Triton X-100 and add it to the PBS (not necessarily autoclaved). Vortex the conical tube to create a homogenous solution. Store at 4 °C.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Triton X-100 | 10% | 5 mL |
| PBS (Recipe 3) | n/a | 45 mL |
| Total | 50 mL |
8. 0.5% Triton X-100 solution
Add the appropriate amount of 10% Triton X-100 solution to PBS (not necessarily autoclaved). Vortex the conical tube to create a homogenous solution. Store at 4 °C.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 10% Triton X-100 | 0.5% | 2.5 mL |
| PBS (Recipe 3) | n/a | 47.5 mL |
| Total | 50 mL |
9. 10% sodium azide solution
Wear a protective mask and gloves when weighing the powder in a fume hood. Vortex the conical tube with sodium azide and dH2O to dissolve the sodium azide. Caution: Sodium azide is toxic.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Sodium azide | 10% (w/v) | 1 g |
| dH2O | n/a | 10 mL |
| Total | 10 mL |
10. BFA solution
Add the DMSO directly into the vessel containing the BFA powder. Vortex the vessel to dissolve the BFA in DMSO. Prepare 50 μL aliquots and store at -20 °C. All steps are carried out in a Class II biological safety cabinet.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BFA | 10 mg/mL | 5 mg |
| DMSO | n/a | 500 μL |
| Total | 500 μL |
11. Nocodazole solution
Add the DMSO directly into the vessel containing the nocodazole powder. Vortex the vessel to dissolve the nocodazole in DMSO. Prepare 50 μL aliquots and store at -20 °C. All steps are carried out in a Class II biological safety cabinet.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Nocodazole | 10 mM | 2 mg |
| DMSO | n/a | 660 μL |
| Total | 660 μL |
12. 1 M calcium chloride solution
Weigh the calcium chloride and put it into a 50 mL conical tube. Add the PBS (not necessarily autoclaved) and vortex the conical tube to dissolve the calcium chloride. Store at RT.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Calcium chloride | 1 M | 1.47 g |
| PBS | n/a | 10 mL |
| Total | 10 mL |
13. 1 M magnesium chloride
Weigh the magnesium chloride and put it into a 50 mL conical tube. Add the PBS (not necessarily autoclaved) and vortex the conical tube to dissolve the magnesium chloride. Store at RT.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Magnesium chloride | 1 M | 2.03 g |
| PBS | n/a | 10 mL |
| Total | 10 mL |
14. 3% PFA
Heat 400 mL of the autoclaved PBS to 60–70 °C in a fume hood while stirring on a hot-plate stirrer. Turn off the fume hood and weigh the PFA. Wear a protective mask and gloves when weighing the powder in a fume hood. Add the PFA powder carefully into the PBS. Clean everything with a damp paper before turning the fume hood back on. Keep the solution heated between 60 and 70 °C. Wait until the PFA has fully dissolved (Tip: Adding a few droplets of 5 M sodium hydroxide helps to dissolve the PFA faster). After the PFA has dissolved, add 1 M calcium chloride and 1 M magnesium chloride. Adjust the PFA solution to the final volume with the remaining PBS. Adjust the pH to 7.4 with either sodium hydroxide or hydrochloric acid. Filter the PFA solution through a Stericup vacuum filter. Prepare aliquots of the PFA solution and store them at -20 °C. These are stable for at least one year.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PFA | 3% | 15 g |
| 1 M calcium chloride (Recipe 12) | 0.1 mM | 50 μL |
| 1 M magnesium chloride (Recipe 13) | 0.1 mM | 50 μL |
| PBS | n/a | 500 mL |
| Total | 500 mL |
Laboratory supplies
1. Tissue culture dish (Nunclon) with lid, polystyrene, radiation, 92 mm × 17 mm (Life Technologies Europe BV, catalog number: 150350)
2. 5 mL sterile serological pipette (Fisher Scientific, catalog number: 11829660)
3. 10 mL sterile serological pipette (Fisher Scientific, catalog number: 11839660)
4. 25 mL sterile serological pipette (Fisher Scientific, catalog number: 11517752)
5. 50 mL conical tubes (Corning, catalog number: 430828)
6. 2 mL microcentrifuge tube (Eppendorf, catalog number: 0030120086)
7. 1.5 mL microcentrifuge tube (Eppendorf, catalog number: 0030120094)
8. 1,000 μL tips (Eppendorf, catalog number: 0030000927)
9. 200 μL tips (Gilson, catalog number: F161930)
10. 10 μL tips (Gilson, catalog number: F161631)
11. 125 μL sterile GRIPTIPS, 5 XYZ racks of 384 tips (Integra Biosciences, catalog number: 6464)
12. 125 μL non-sterile GRIPTIPS, 5 XYZ racks of 384 tips (Integra Biosciences, catalog number: 6463)
13. 10 mL disposable reservoirs, sterile, SureFloTM anti-sealing array (Integra Biosciences, catalog number: 4373)
14. 300 mL robotic reservoir, flat bottom, sterile (Thermo Scientific, Nunc, catalog number: 10723363)
15. Scepter 2.0 handheld automated cell counter (Merck, catalog number: PHCC20060)
16. Scepter 60 μm sensors (Merck, catalog number: PHCC60050)
17. Pasteur pipette, glass unplugged, 230 mm (Fisher Scientific, catalog number: 1156-6963)
18. Stericup quick release vacuum filtration system (Merck, catalog number: S2HVU05RE)
19. CYTOO plates 96RW custom fibronectin-coated, DC45/P300 (CYTOO, catalog number: 20-950-10); store at 4 °C
Equipment
1. Class II biological safety cabinet (Faster)
2. CO2 incubator (New Brunswick Scientific, model: Innova CO-170)
3. Vacusafe vacuum aspirator with Vacuboy (Integra Biosciences, catalog numbers: 158300 and 155500)
4. Pipetboy Pro (Integra Biosciences)
5. Research Plus variable adjustable volume pipettes, volume: 100–1,000 μL (Eppendorf, catalog number: 3123000063)
6. Research Plus variable adjustable volume pipettes, volume: 20–200 μL (Eppendorf, catalog number: 3123000055)
7. Research Plus variable adjustable volume pipettes, volume: 2–20 μL (Eppendorf, catalog number: 3123000039)
8. Research Plus variable adjustable volume pipettes, volume: 0.5–10 μL (Eppendorf, catalog number: 3123000020)
9. Waterbath (Grant, model: JB nova)
10. Light microscope (Olympus, model: CKX53); objectives: 10× and 20×
11. Viaflo 96 (with 125 μL head and 2 spring-loaded plate holders) (Integra Biosciences, catalog number: 6001)
12. Viaflo electronic pipette 8-channel, 5–125 μL (Integra Biosciences, catalog number: 4722)
13. Laboratory centrifuge with rotor adaptor for 15 and 50 mL conical tubes (Eppendorf, model: 5810 R, catalog number: 5811000065)
14. Vortex (Merck, catalog number: Z258423)
15. Mini microfuge (VWR)
16. Tube racks
17. Hot-plate stirrer
18. Opera Phenix high-content screening system (Revvity)
Software and datasets
1. Harmony (Revvity, Version 4.8 and 4.9); requires a license
2. All image data relevant to this paper are available from the BioImage Archive (https://www.ebi.ac.uk/biostudies/bioimages/studies) at DOI:10.6019/S-BIAD1259
Procedure
文章信息
稿件历史记录
提交日期: Feb 6, 2025
接收日期: May 6, 2025
在线发布日期: May 22, 2025
出版日期: Jun 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Mysior, M. M. and Simpson, J. C. (2025). An Automated Imaging Method for Quantification of Changes to the Endomembrane System in Mammalian Spheroid Models. Bio-protocol 15(11): e5331. DOI: 10.21769/BioProtoc.5331.
分类
细胞生物学 > 细胞分离和培养 > 3D细胞培养
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














