- EN - English
- CN - 中文
Synchronized Visualization and Analysis of Intracellular Trafficking and Maturation of Orthoflavivirus Subviral Particles
Orthoflavivirus亚病毒颗粒胞内运输与成熟过程的同步可视化与分析
发布: 2025年05月20日第15卷第10期 DOI: 10.21769/BioProtoc.5324 浏览次数: 2140
评审: Keisuke TabataLuis Alberto Sánchez VargasAnonymous reviewer(s)
Abstract
Orthoflavivirus is an enveloped, positive-stranded RNA virus that buds into the endoplasmic reticulum (ER) lumen. The budded virus particles are subsequently transported to the Golgi apparatus and secreted into the extracellular environment via the conventional secretion pathway. In this protocol, we describe a method for monitoring the secretion of Orthoflavivirus particles from the ER. To visualize intracellular membrane trafficking, we combine two distinct imaging techniques: the retention using selective hooks (RUSH) system and the split green fluorescent protein (GFP) system. In this approach, GFP11, a peptide tag fused to prME, the outer coat structural protein of Japanese encephalitis virus particles, was co-expressed in HeLa cells along with two additional components: GFP1-10 fused to a streptavidin-binding peptide and a hook construct consisting of streptavidin fused to the ER retention sequence KDEL. Time-lapse imaging was performed after the addition of biotin, which releases the captured GFP-labeled subviral particles from the ER. This method enables synchronized visualization of intracellular subviral particle trafficking and serves as a valuable tool for analyzing the maturation process of Orthoflavivirus particles within cells.
Key features
• Synchronized intracellular movement of Orthoflavivirus particles is visualized by the retention using selective hooks (RUSH) system.
• Split GFP system is used to label viral particles.
• This protocol has broader applications in investigating the transport of secretory proteins, especially those that are challenging to tag with full-length fluorescent proteins.
Keywords: Retention using selective hooks (RUSH) (选择性锚定保留系统(RUSH))Graphical overview
Background
Orthoflaviviruses (renamed in 2023, formerly known as flaviviruses) are enveloped, positive-stranded RNA viruses that include several human pathogens, such as Japanese encephalitis virus (JEV), Dengue virus, Zika virus, West Nile virus, and yellow fever virus. The virus particles are approximately 40–60 nm in diameter and consist of genomic RNA encapsulated by capsid proteins within a lipid bilayer. Two proteins are anchored on the outer side of the membrane: precursor membrane protein/membrane protein (prM/M) and envelope protein (E) [1,2]. Virions are considered to bud into the endoplasmic reticulum (ER) lumen, from where they are subsequently transported to the extracellular environment via the conventional secretory pathway [3]. In addition to the virion assembly and secretion pathway, empty particles (subviral particles, SVPs), formed by prM/M and E proteins and lacking the nucleocapsid, are also released from cells through the same secretory pathway as virions [4]. During the budding and secretion of these particles, prM and E undergo several post-translational modifications, including N-linked glycosylation, processing of pr and M proteins, conformational changes, and re-assembly [3,5].
The tracking of membrane vesicle transport within cells has been extensively studied, revealing the dynamics of secretory proteins in living cells. Membrane trafficking, which involves enveloped cargo proteins, primarily begins in the ER lumen and proceeds in an anterograde direction to various Golgi regions (cis, medial, trans, and the trans-Golgi network), which serve as sorting and modification stations [6]. In these sorting zones, the fate of cargo vesicles is determined, directing them to their final destination. Recently, an intriguing approach was developed for tracking protein secretion: the retention using selective hooks (RUSH) system. This elegant methodology enables the study of synchronized cargo trafficking by observing fluorescent-tagged vesicles in cells [7,8]. The RUSH system consists of two key components: (1) a protein of interest (POI) tagged with a fluorophore and a streptavidin-binding peptide (SBP) and (2) a streptavidin molecule bound to a retention molecule (the "hook") in a specific cellular compartment. In the absence of biotin, these two molecules form a complex that is retained in the donor compartment. However, when biotin is added to the culture medium, the POI–fluorophore–SBP complex is released into the next compartment. This transition allows for time-dependent tracking of the POI using fluorescence microscopy [7].
The GFP11 peptide (amino acid sequence: RDHMVLHEYVNAAGIT) is a part of the split-GFP system that can reconstitute the intact superfolder GFP when paired with the GFP1-10 fragment. As a result, the presence of GFP1-10 and excitation light facilitates the detection of the GFP11 peptide [9]. Since GFP11 is a short peptide tag consisting of only 16 amino acids, it minimally affects the function of the fused POI and its complexes. Screening was conducted to identify positions on the viral particle surface where the insertion of a short peptide would not interfere with the folding of prME proteins or SVP assembly, and the 275 aa loop position was found to be suitable [10]. We inserted the GFP11 peptide at this position and demonstrated that the co-expression of the GFP1-10 fragment facilitates GFP labeling of JEV particles [11].
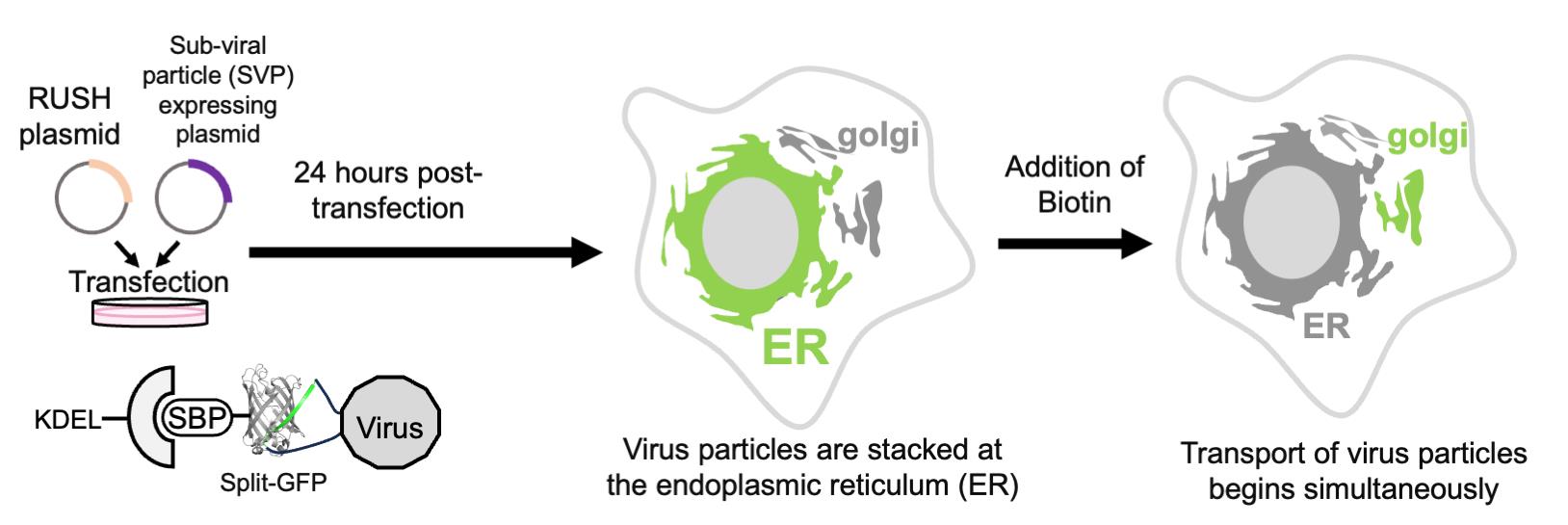
Based on the combination of RUSH and split-GFP methodologies, we present a protocol that enables the quantitative evaluation of viral particle transport as cargo in a time-dependent manner (Figure 1). Using this system, we confirmed that SVPs formed in the ER were transported to the Golgi apparatus within 30 min of biotin addition (Figure 2). This protocol is not only valuable for studying orthoflavivirus particle secretion but also has broader applications in investigating the transport of secretory proteins, especially those that are challenging to tag with full-length fluorescent proteins (e.g., GFP, mCherry).
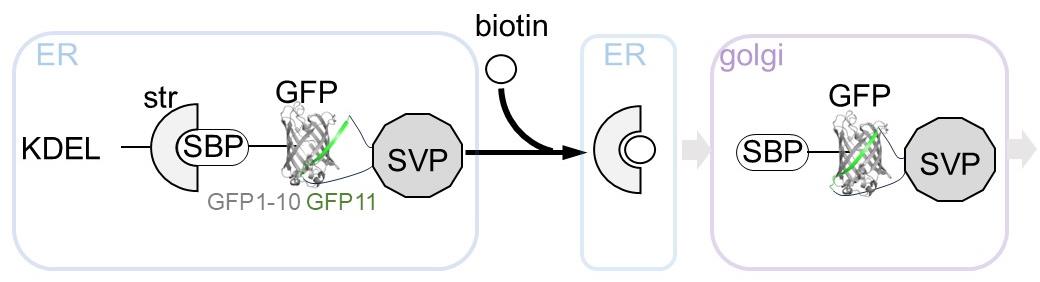
Figure 1. Overview of the retention using selective hooks (RUSH)-based subviral particle (SVP) secretion assay. The schematic illustration represents the RUSH system for SVPs labeled with the split-GFP system. The addition of biotin simultaneously releases the ER-retained GFP-labeled SVPs, enabling synchronized tracking of SVP transport dynamics within cells. Abbreviations used in the figure: ER, endoplasmic reticulum; KDEL, ER retention sequence; Str, streptavidin; SBP, streptavidin-binding peptide; GFP, green fluorescent protein (reconstituted by GFP1-10 and GFP11 peptide); SVP, subviral particle; Golgi, Golgi apparatus.
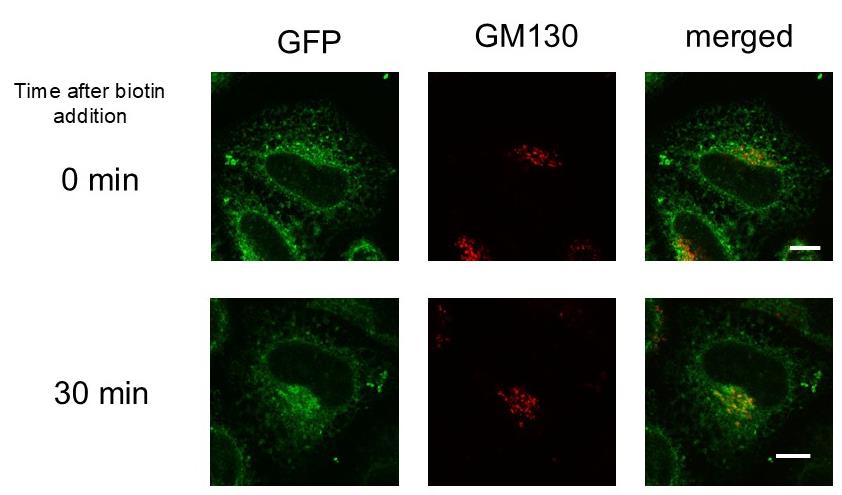
Figure 2. Transport of subviral particles (SVPs) from the endoplasmic reticulum (ER) to the Golgi apparatus. HeLa cells transfected with two plasmids, Str-KDEL_IL-2ss-HiBiT-SBP-GFP1-10 and pCAG-JEprME-S275-GS-GFP11-GS-S276, were treated with 400 μM biotin. Cells were fixed and stained with anti-GM130 either before the addition of biotin (upper panels) or 30 min after its addition (lower panels). Green fluorescent protein (GFP) signals (green, left) and GM130 signals (red, middle) were visualized using confocal laser microscopy. Merged images are shown in the right panels. Scale bar = 10 μm.
Materials and reagents
Note: The materials used in this study can be obtained from the corresponding author, Eiji Morita, upon reasonable request.
Biological materials
1. HeLa cells (ATCC, catalog number: CRM-CCL-2)
Note: HeLa cells, passaged at least 50 times in our laboratory, were used. To prevent mycoplasma contamination, cells were stained with Hoechst to check for intracellular DNA, which indicates contamination. Additionally, cells were periodically treated with BM-Cyclin (Roche, catalog number: 10799050001) to remove mycoplasma contamination.
2. Plasmid Str-KDEL_IL-2ss-HiBiT-SBP-GFP1-10 (V206K)
Notes:
1. This plasmid was constructed by replacing the TNF-SBP-EGFP sequence of Str-KDEL_TNF-SBP-EGFP with an in-frame tandem sequence of interleukin-2 signal peptide, HiBiT tag, SBP, and GFP1-10 (V206K).
2. Str-KDEL_TNF-SBP-EGFP is one of the original RUSH constructs reported by Boncompain et al. [7] and is available from Addgene (#65278). RUSH constructs are mammalian expression constructs that tandemly encode a hook protein (Str-KDEL in this case) and a reporter protein [GOI, interleukin-2 signal peptide-HiBiT-SBP-GFP1-10 (V206K) in this case] linked via an internal ribosome entry site sequence. We selected Str-KDEL as the hook protein based on our experimental requirements (see General note 1).
3. The interleukin-2 signal peptide and HiBiT tag sequences were introduced via overlap polymerase chain reaction (PCR). The interleukin-2 signal peptide and SBP sequence was amplified using Ii-Str_ssSBP-EGFP (Addgene #65277) as a template, and the GFP1-10 (V206K) sequence was amplified using ZipGFP1-10_TEV (Addgene #81242) as a template (see General notes 2 and 3). These fragments were then joined using overlap PCR. The resulting DNA fragment was inserted into the AscI/PacI site of Str-KDEL_TNF-SBP-EGFP (see General note 4).
3. Plasmid pCAG-JEprME-S275-GS-GFP11-GS-S276
Notes:
1. This plasmid was constructed by inserting a sequence encoding a GFP11-containing peptide into the E gene of pCAG-prME.
2. The pCAG-prME plasmid, provided by Dr. Ryosuke Suzuki, was generated in a previous study [12]. This plasmid contains the carboxyl-terminal 22 residues of the JEV Nakayama strain C protein (corresponding to the prM protein signal sequence), the full-length prM protein, and the full-length E protein. For detailed information on the pCAG-prME sequence, please contact Dr. Ryosuke Suzuki (National Institute of Infectious Diseases, Japan).
3. A GFP11 peptide sequence, flanked by GS linker sequences at both ends, was inserted in-frame between amino acid residues S275 and S276 of the JEV E protein. The permissive insertion site within the JEV E protein was previously determined via a Flag-tag insertion screening study [10]. For the full-length amino acid sequence of the E protein, refer to Saga et al. [10] (see General notes 5 and 6).
4. Overlap PCR was performed using pCAG-prME as a template with primers containing this sequence, and the GFP11 peptide coding sequence was inserted into the E gene. The resulting DNA fragment was then inserted into the KpnI/XhoI sites of pCAG-MCS2, a pCAGGS [13] derived plasmid vector containing additional multiple cloning sites (see General note 7).
Reagents
1. DMEM (4.5 g/L glucose) without L-Gln, sodium pyruvate, and phenol red, liquid (Nacalai, catalog number: 08489-45)
2. Fetal bovine serum (FBS) (Thermo, catalog number: 10270-106)
3. 100 mM sodium pyruvate solution (100×) (Nacalai, catalog number: 06977-34)
4. 200 mM L-Glutamine stock solution (Nacalai, catalog number: 16948-04)
5. Phosphate-buffered saline without calcium and magnesium (PBS) (Nacalai, catalog number: 14249-24)
6. Lipofectamine 3000 transfection reagent (Thermo, catalog number: L3000015)
7. OptiMEM (Thermo, catalog number: 31985062)
8. 100 mM biotin stock solution (IBA GmBH, catalog number: 6-6325-001)
9. Sodium hydroxide (Nacalai, catalog number: 31511-05)
10. Hydrochloric acid (Nacalai, catalog number: 18320-15)
11. Paraformaldehyde (Nacalai, catalog number: 26126-25)
12. Triton X-100 (Nacalai, catalog number: 35501-02)
13. Anti-GM130 antibody (BD Biosciences, catalog number: 610822)
14. Anti-mouse IgG antibody, Alexa Fluor 594 conjugated (Jackson ImmunoResearch, catalog number: 115-585-146)
15. Fluoromount-G (Southern Biotechnology Associates, catalog number: 0100-01)
16. MGK-S (Matsunami, catalog number: FX00100)
Solutions
1. Culture media (see Recipes)
2. 1 N NaOH solution (see Recipes)
3. 4% PFA (paraformaldehyde) (see Recipes)
4. 10% TX-100 (w/v) solution (see Recipes)
5. Permeabilizing and blocking solution (see Recipes)
Recipes
1. Culture media
a. 500 mL of DMEM.
b. Add 50 mL of FBS, heat-inactivated by incubation at 56 °C for 45 min.
c. Add 5 mL of 100 mM sodium pyruvate solution (100×).
d. Add 5 mL of 200 mM L-glutamine stock solution.
e. Store at 4 °C.
2. 1 N NaOH solution
a. For 500 mL of 1 N NaOH solution, add 350 mL of Milli-Q water to a glass beaker.
b. Add 20 g of sodium hydroxide drops to Milli-Q water.
c. After completely dissolved, adjust the volume of the solution to 500 mL with water.
d. Transfer the solution to a plastic bottle and store it at room temperature.
3. 4% PFA
a. For 1 L of 4% paraformaldehyde (formaldehyde), add 800 mL of PBS to a glass beaker on a stir plate in a ventilated hood. Heat while stirring to approximately 60 °C. Ensure that the solution does not boil.
b. Add 40 g of paraformaldehyde powder to the heated PBS solution.
c. The powder will not immediately dissolve into the solution. Slowly raise the pH by adding 1 N NaOH dropwise using a pipette until the solution clears.
d. Once the paraformaldehyde is dissolved, cool and filter the solution.
e. Adjust the volume of the solution to 1 L with PBS.
f. Recheck the pH and adjust it to approximately 6.9 with small amounts of diluted hydrochloric acid.
Note: To succeed in the downstream process after cell fixation, please pay attention to the pH of the PFA solution.
g. The solution can be aliquoted and frozen or stored at 2–8 °C for up to one month.
4. 10% TX-100 (w/v) solution
a. For 50 mL of 10% TX-100 solution, add 30 mL of Milli-Q water to a 50 mL tube.
b. Add 5 g of Triton X-100 to Milli-Q water and rotate the tube overnight.
c. After the Triton X-100 is dissolved completely, adjust the volume of the solution to 50 mL with Milli-Q water.
d. Store at room temperature.
5. Permeabilizing and blocking solution
a. Dilute 10% TX-100 solution with PBS by 1:100 (v/v). The final TX-100 concentration is 0.1% (w/v).
b. Dilute FBS with PBS by 1:10 (v/v). The final FBS concentration is 10% (v/v)
Laboratory supplies
1. 35 mm glass-bottom dish (Phoenix Science, catalog number: P35G-0-20-C)
2. Humidified incubator (37 °C, 5% CO2) (PHCbi)
3. 1.5 mL tube (Watson, catalog number: 131-815C)
4. Glass-bottom dish (MatTek Life Science, model: P35G-1.5-14-C)
5. Micro cover glass (Matsunami, thickness: No1S, diameter: 12 mm)
6. White glass, frosted microscope slides (Matsunami, catalog number: S2441)
Equipment
1. Microscope for live imaging based on:
a. EVIDENT IX83 P2ZF inverted fluorescence microscope (Evident, Tokyo, Japan)
b. DP80 CCD digital camera (Evident)
c. IX3-ZDC2 Z-drift compensator (Evident)
d. UPLXAPO 60×/1.35 numerical-aperture oil-immersion objective (Evident, catalog number: UPLXAPO60XO)
e. U-FUNA/U-FBNA/U-FMCHE filter set (Evident)
f. Stage top incubation system (Tokai Hit)
2. Microscope for fixed samples based on:
a. EVIDENT IX83 P2ZF inverted fluorescence microscope (Evident)
b. FV3000 confocal laser scanning microscope (high-speed resonant scanner) (Evident)
c. UPLXAPO 60×/1.35 numerical-aperture oil-immersion objective (Evident, catalog number: UPLXAPO60XO)
d. 5-color laser combiner (Coherent)
Software and datasets
1. cellSens Dimension (Evident)
2. ImageJ/Fiji
Procedure
文章信息
稿件历史记录
提交日期: Feb 12, 2025
接收日期: Apr 27, 2025
在线发布日期: May 8, 2025
出版日期: May 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Ishida, K. and Morita, E. (2025). Synchronized Visualization and Analysis of Intracellular Trafficking and Maturation of Orthoflavivirus Subviral Particles. Bio-protocol 15(10): e5324. DOI: 10.21769/BioProtoc.5324.
分类
微生物学 > 微生物细胞生物学 > 细胞染色
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













