- EN - English
- CN - 中文
Development of Polyethylene Glycol Diacrylate-Based Micropattern Substrate to Study the Interplay Between Surface Topography and Cellular Response for Tissue Engineering Applications
基于PEGDA的微图案化底物构建及其在组织工程中表面形貌与细胞响应关系研究中的应用
发布: 2025年05月20日第15卷第10期 DOI: 10.21769/BioProtoc.5323 浏览次数: 2579
评审: Rajesh RanjanEVANGELOS THEODOROUAnonymous reviewer(s)
Abstract
A key goal in the bioengineering field is the development of surface patterning of proteins that guide and control cellular organization. To this end, we developed a method to create a microstructured hydrogel based on soft-lithography techniques using polydimethylsiloxane (PDMS) and polyethylene glycol diacrylate (PEGDA). This approach involves the design of microfluidic geometries using graphical software, employing PDMS as a mold and leaving PEGDA as a substrate for the fabrication of microstructures and, thus, patterning extracellular matrix (ECM) proteins to promote cell adhesion. The combination of these techniques allows the fabrication of hydrogel microstructures without following conventional photolithography methods, such as the use of a photomask, the alignment required to produce the patterns, and the associated expenses. This study highlights the versatility and potential of PEGDA-based hydrogels as platforms to advance tissue engineering strategies.
Key features
• This protocol focuses on investigating the feasibility of patterning PEGDA as a substrate for protein surface patterning and further tissue engineering applications.
• Optimization of the fabrication of PEGDA hydrogels into simple shapes and angular patterns, ensuring a robust substrate capable of guiding cellular responses.
Keywords: PEGDA (PEGDA)Graphical overview
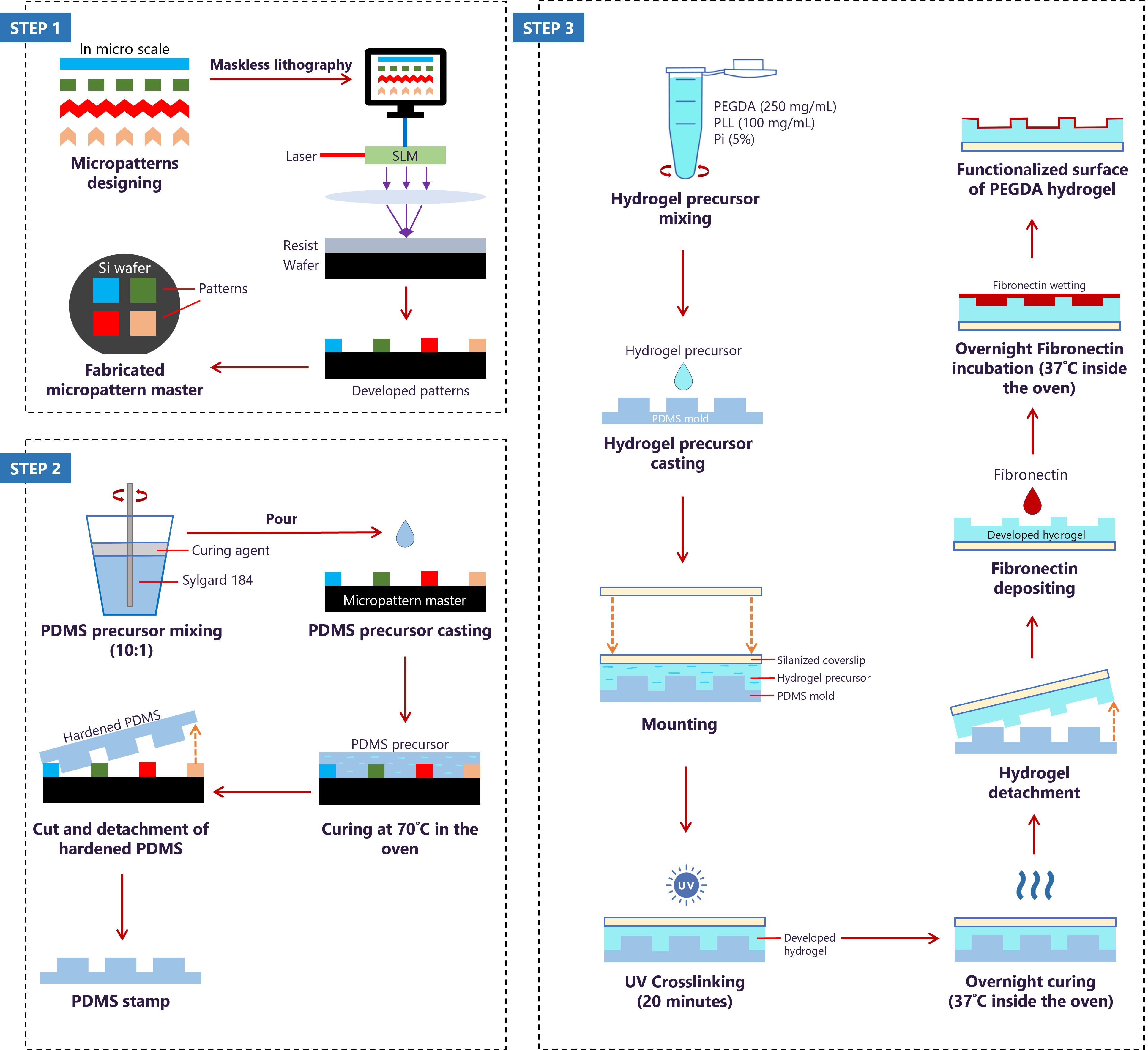
Fabrication of biofunctionalized microtopography hydrogels using soft lithography and fibronectin-deposition approach. The protocol consists of designing micropatterns for the fabrication of the silicon (Si) wafer using QCAD graphical software (step 1), followed by mixing and casting the PDMS precursor on the Si wafer to obtain PDMS molds with the different micropatterns (step 2). Finally, developing PEGDA hydrogel on the PDMS mold to transfer micropatterns to the hydrogel and depositing fibronectin on the hydrogel’s surface to functionalize it (step 3).
Background
Central to the new era of tissue engineering is the development of biofunctionalized scaffolds where engineered structures are designed to mimic the intricate extracellular matrix (ECM) and support cellular growth [1–4]. Collaborative efforts by material scientists, electronic engineers, and cell biologists have led to cutting-edge techniques, such as microfabrication and nanotechnology, to create scaffolds that can closely imitate the native ECM to guide cell behaviour [1–6]. Although the interconnected roles of biochemical and biophysical cues are widely recognized [7–9], researchers often focus on biochemical aspects, overlooking the importance of biophysical cues in guiding cell behaviour. Hydrogel substrates have been created through pattern transfer using deep UV lithography and/or soft lithography, with a focus on fabricating polyethylene glycol diacrylate (PEGDA)-based hydrogels. Polyethylene glycol (PEG), being inherently inert to protein adsorption and cellular attachment, is considered ideal for anti-fouling coatings in biomedical applications and medical devices [17,18]. Linear PEG can be chemically modified into a reactive polymeric mesh network with a diacrylate (DA) crosslinker [10], making PEGDA suitable for various applications, such as drug delivery systems, tissue engineering, and wound healing [11–15]. The utility of PEGDA lies in its tunability of its mechanical and physico-chemical properties [10,16,17]. This versatility allows for precise control over the PEGDA’s characteristics, such as shape, stiffness, swelling behaviour, and degradation rate, making it an excellent choice for creating customized materials, including specific patterns and topographies [10,16,17]. However, since PEGDA remains inert to cell attachment, the coating of fibronectin, homogeneously or in selective PEGDA regions, enhances its bio-functionality and promotes cell adhesion on the PEGDA surface. Overall, the feasibility of creating and using patterned PEGDA allows for the optimization of surface properties to guide cellular organization, advancing its applications in tissue engineering.
Materials and reagents
Biological materials
1. C2C12 cell line (ATCC, catalog number: CRL-1772)
2. Fetal bovine serum (FBS) (Sigma Life Science, catalog number: F7524)
3. Fibronectin (Sigma-Aldrich, catalog number: F1141)
4. Fibrinogen (Thermo Fisher Scientific, catalog number: F35200)
Reagents
1. Dulbecco’s modified Eagle medium (DMEM) supplemented with 4.5 g/L D-glucose, L-glutamine, and pyruvate (Gibco, catalog number: 41966-029)
2. Penicillin/Streptomycin (Thermo Fisher Scientific, catalog number: 15140122)
3. Trypsin-EDTA (0.25%), phenol red (Thermo Fisher Scientific, catalog number: 25200072)
4. 1× phosphate saline buffer (PBS) (Thermo Fisher Scientific, catalog number: 14190169)
5. Trypan blue (Invitrogen, catalog number: T10282)
6. Alamar blue (Thermo Fisher Scientific, catalog number: DAL1025)
7. Absolute ethanol (Carl Roth, catalog number: 9065.5)
8. Acetic acid (Honeywell, catalog number: 607-002-00-6)
9. 3-(Trimethoxysily) propyl methacrylate (Silane A174) (Sigma-Aldrich, catalog number: 440159)
10. Polyethylene glycol diacrylate (PEGDA) (MW = 700) (Sigma-Aldrich, catalog number: 455008)
11. Poly(L-lysine) (PLL) (Biosynth, catalog number: FP14985)
12. PLPP gel photoinitiator (Alvéole)
13. 70% ethanol
14. Isopropanol
15. Acetone
16. Sylgard 184 elastomer base and curing agent (VWR, catalog number: 634165S)
17. 4% paraformaldehyde (PFA)
18. Phalloidin-TRITC (Sigma-Aldrich, catalog number: P1951)
19. Hoechst (Thermo Fisher Scientific, catalog number: H1398)
20. Mowiol (Carl Roth, catalog number: 0731.2)
21. Sodium hydroxide solution (NaOH) (lab-made)
22. Ultrapure water (lab-made)
23. Bovine serum albumin (BSA) powder (Sigma-Aldrich, catalog number: A2153)
24. 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: C-40020)
Solutions
1. Complete cell culture medium (see Recipes)
2. Sodium hydroxide solution (see Recipes)
3. PLL mix solution (see Recipes)
4. PEGDA mix solution (150 mg/mL) (see Recipes)
5. PEGDA mix solution (250 mg/mL) (see Recipes)
6. PEGDA mix solution (350 mg/mL) (see Recipes)
7. Hydrogel precursor solution (see Recipes)
8. Alamar blue solution (see Recipes)
9. Silanization immersion solution (see Recipes)
10. HEPES solution (see Recipes)
11. Micropattern labeling solution (see Recipes)
12. 5% BSA solution (see Recipes)
13. 1% BSA solution (see Recipes)
14. PDMS solution (see Recipes)
15. Immunostaining solution (see Recipes)
Recipes
1. Complete cell culture medium (50 mL)
Storage: 4 °C, up to 3 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM | n/a | 44.5 mL |
| FBS | 10% (v/v) | 5 mL |
| Penicillin/streptomycin | 1% (v/v) | 0.5 mL |
| Total | n/a | 50 mL |
2. Sodium hydroxide solution (100 mL, 0.15 M, pH 7.8)
Storage: room temperature, up to 6 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaOH solution (1 M) | 0.15 M | 15 mL |
| Ultrapure water | n/a | 85 mL |
| Total | n/a | 100 mL |
3. PLL mix solution (100 mg/mL)
Immediate use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PLL powder | 100 mg/mL (w/v) | 100 mg |
| NaOH solution (0.15 M) | n/a | 1 mL |
| Total | n/a | 1 mL |
4. PEGDA mix solution (150 mg/mL)
Immediate use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PEGDA solution (1 M) | 150 mg/mL (v/v) | 26.8 μL |
| Ultrapure water | n/a | 173.2 μL |
| Total | n/a | 200 μL |
5. PEGDA mix solution (250 mg/mL)
Immediate use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PEGDA solution (1 M) | 250 mg/mL (v/v) | 44.6 μL |
| Ultrapure water | n/a | 155.4 μL |
| Total | n/a | 200 μL |
6. PEGDA solution (350 mg/mL)
Immediate use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PEGDA solution (1 M) | 350 mg/mL (v/v) | 62.5 μL |
| Ultrapure water | n/a | 137.5 μL |
| Total | n/a | 200 μL |
7. Hydrogel precursor solution (0.42 mL)
Immediate use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PEGDA mix solution | 150/250/350 mg/mL (v/v) | 200 μL |
| PLL mix solution | 100 mg/mL (w/v) | 200 μL |
| PLPP gel photoinitiator | 5% | 20 μL |
| Total | n/a | 420 μL |
8. Alamar blue solution (100 μL)
Immediate use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Alamar blue | 10% | 10 μL |
| Complete culture medium | n/a | 90 μL |
| Total | n/a | 100 μL |
9. Silanization immersion solution (300 mL)
Immediate use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ultrapure water | n/a | 10 mL |
| Acetic acid | 1 (ratio) | 18.75 mL |
| Silane A174 | 1 (ratio) | 18.75 mL |
| Absolute ethanol | 14 (ratio) | 252.5 |
| Total | n/a | 300 mL |
10. HEPES solution (500 mL)
Storage: 4 °C, up to 3 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| HEPES (1 M) | 10 mM | 5 mL |
| Ultrapure water | n/a | 495 mL |
| Total | n/a | 500 mL |
11. Micropattern labeling solution (0.7 mL)
Immediate use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Fibronectin | 50 μg/mL | 17.5 μL |
| Fibrinogen | 10 μg/mL | 4.7 μL |
| HEPES solution (10 mM) | n/a | 677.8 μL |
| Total | n/a | 700 μL |
12. 5% BSA solution (50 mL)
Storage: 4 °C, one week.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA powder | 5% (w/v) | 2.5 g |
| 1× PBS | n/a | 50 mL |
| Total | n/a | 50 mL |
13. 1% BSA solution (50 mL)
Storage: 4 ˚C, one week.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA powder | 1% (w/v) | 0.5 g |
| 1× PBS | n/a | 50 mL |
| Total | n/a | 50 mL |
14. PDMS solution (50 mL)
Storage: -20 ˚C, up to 5 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sylgard 184 elastomer base | 10 (ratio) | 50 g |
| Curing agent | 1 (ratio) | 5 g |
| Total | n/a | 55 g |
15. Immunostaining solution (0.7 mL)
Immediate use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Phalloidin | 1:200 | 3.5 μL |
| Hoechst | 1:1,000 | 0.7 μL |
| 5% BSA | n/a | 695.8 μL |
| Total | n/a | 700 μL |
Laboratory supplies
1. T-75 cell culture flask (Fisher Scientific, catalog number: 50-809-260)
2. Glass bottom 6-well plate (lab custom-made)
3. 96-well plate (Fisher Scientific, catalog number: 92096)
4. 6-well plate (Fisher Scientific, catalog number: 92006)
5. Hemocytometer (Sigma-Aldrich, catalog number: Z359629-1EA)
6. 20 × 20 mm2 glass coverslip (Carl Roth, catalog number: H873.2)
7. 18 × 18 mm2 glass coverslip (Carl Roth, catalog number: 0657.2)
8. Teflon rack (custom-made)
9. Glass box (custom-made)
10. 20 mL syringe (B. Braun Inkjet, catalog number: 4606736V)
11. 50 mm length needle (B. Braun Sterican, catalog number: 466 5503)
12. 1.5 mL microcentrifuge tubes (Axygen, catalog number: 10620934)
13. 0.6 mL microcentrifuge tubes (Nest Biotechnology, catalog number: 605001)
14. 15 mL conical tube (Fisher Scientific, catalog number: 20160618-058)
15. 50 mL conical tube (Cellstar, catalog number: 227261)
16. 5 mL serological pipette (Eppendorf, catalog number: 0030127714)
17. 10 mL serological pipette (Eppendorf, catalog number: 0030127722)
18. 25 mL serological pipette (Eppendorf, catalog number: 0030127730)
19. Aluminum foil (Reynolds Consumer Products LLC, catalog number: 1090000084)
20. Chromium mask (Toppan Photomasks, Inc.)
21. Micropattern master (IMSEAM, Heidelberg)
22. 100 mm diameter Petri dish (Corning, catalog number: 430167)
23. 60 mm diameter Petri dish (Falcon, catalog number: 353004)
24. 35 mm diameter Petri dish (Falcon, catalog number: 353001)
25. Spatula
26. Scalpel No. 11 (Fisher Scientific, catalog number: 11708353)
27. 76 mm × 26 mm microscope slides (Marienfeld, catalog number: 1000200)
Equipment
1. Incubator (Thermo Scientific, model: BBD 6220)
2. Automation cell Countess II FL (Invitrogen, model: AMQAF1000)
3. Multi-mode microplate reader (Tecan, model: Spark)
4. Nitrogen gun (MPI)
5. UV crosslinker chamber (Vilber Lourmat, model: BLX312)
6. Ultraviolet-ozone cleaner machine (Jetlight Company Inc., model: 342-220)
7. Vortex machine (StarLab, model: N2400-6110)
8. Fume hood (Thermo Fisher Scientific, model: EN 12469)
9. Weighing balance (Kern, model: AEJ 200-5CM)
10. Centrifuge machine (VWR, model: Mega Star 600R)
11. Desiccator (Duran, model: DN150)
12. -20 ˚C blast freezer (Liebherr, model: MediLine)
13. Oven (VWR, model: INCU Line)
14. Optical microscope (Zeiss, model: Primovert)
15. Optics11 Chiaro Nanoindeter (Optics11 Life)
16. Delta Vision (Olympus)
Software and datasets
1. QCAD software (3.31.1, 19.04.2024)
2. ImageJ software (1.8.0, 07.06.2024)
3. GraphPad Prism (8.4.3, 02.09.2024)
4. Optics11 Life DataViewer (V2, 31.07.2024)
Procedure
文章信息
稿件历史记录
提交日期: Feb 24, 2025
接收日期: Apr 18, 2025
在线发布日期: May 8, 2025
出版日期: May 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Darwis, M. K. A., Levario-Diaz, V., Pashapour, S., Voigt, J. L., Lebaudy, E., Sabani, N., Bakar, A. S. A., Vrana, N. E., Lavalle, P., Cavalcanti-Adam, E. A. and Ngalim, S. H. (2025). Development of Polyethylene Glycol Diacrylate-Based Micropattern Substrate to Study the Interplay Between Surface Topography and Cellular Response for Tissue Engineering Applications. Bio-protocol 15(10): e5323. DOI: 10.21769/BioProtoc.5323.
分类
细胞生物学 > 细胞工程 > 组织工程
生物工程 > 生物医学工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














