- EN - English
- CN - 中文
Quantifying Thrombogenicity: A Bioanalytical Protocol for the Absorbance-Based Assessment of Vascular Implants with Plasma
血栓形成能力的定量分析:基于吸光度的血浆评估血管植入物的生物分析方案
(*contributed equally to this work) 发布: 2025年05月20日第15卷第10期 DOI: 10.21769/BioProtoc.5316 浏览次数: 1554
评审: Elena A. OstrakhovitchEVANGELOS THEODOROU
Abstract
Assessing thrombogenicity is crucial for evaluating biomaterials, especially those that interface with flowing blood, such as cardiovascular implants, including vascular stents, grafts, and stent-grafts. To standardize thrombogenicity assessments, we use human plasma and quantify the light absorbance associated with the biomaterial. For this evaluation, various tubular vascular implants from leading brands—such as bare-metal stents, drug-eluting stents, vascular grafts, and stent-grafts—are longitudinally sectioned into small pieces and placed in a low-adhesion 96-well plate. Using either platelet-rich plasma (PRP) or platelet-poor plasma (PPP), we measure the absorbance of light passing through the plate over an hour and plot the resulting curve. This method quantifies the thrombogenicity of a biomaterial under controlled conditions. Key factors examined include anticoagulants, platelet presence, and surface-coating molecules to assess their impact on thrombogenicity. Using this simple, reproducible protocol, we demonstrated (a) the relative efficacy of various anticoagulants in thrombogenicity assessments, and (b) the effectiveness of vascular coating molecules in reducing thrombogenicity on stents. This streamlined approach offers valuable insights for optimizing biomaterial performance in vascular implants. Unlike conventional clotting assays, which focus on standardized blood clotting mechanisms, this assay is tailored to evaluate biomaterials and external parameters influencing thrombogenicity.
Key features
• This protocol is a static, in vitro, quantitative assessment of thrombogenicity for both novel and commercial vascular biomaterials with flat or tubular geometries.
• Thrombogenicity is assessed by evaluating the opaqueness of platelet-rich or platelet-poor plasma over time to measure fibrin formation.
• Various anticoagulants such as sodium citrate, sodium ethylenediaminetetraacetic acid, and sodium heparin are compared to evaluate the efficacy of the protocol.
• This protocol compares the thrombogenicity of various commercial stents, grafts, and stent-graft hybrids, as well as novel molecular coatings.
Keywords: Platelet-rich plasma (PRP) (富血小板血浆(PRP))Graphical overview
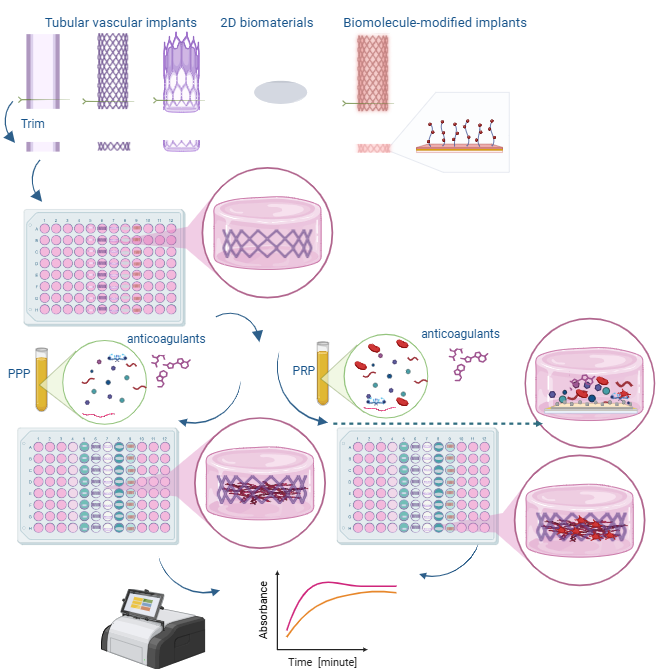
Overview of the mechanisms and steps performed in this protocol
Background
Cardiovascular diseases are the leading cause of death worldwide, with coronary artery disease, cerebrovascular disease, and peripheral vascular disease being the most prevalent. These conditions are often managed using vascular stents or grafts, but thrombosis remains a significant challenge, contributing to the failure of these devices. As a result, patients with stents or grafts typically require blood thinners, which can cause serious side effects. Therefore, minimizing thrombogenicity is a critical focus for both commercially available stents and grafts and the development of new vascular biomaterials.
The surfaces of vascular biomaterials interact with blood components to initiate coagulation through the intrinsic clotting cascade. To help minimize the risk of thrombogenesis, anticoagulants such as sodium citrate, sodium ethylenediaminetetraacetic acid (EDTA), and sodium heparin are often used. These anticoagulants are capable of chelating calcium ions, reducing the concentration of free calcium in the blood, and thus inhibiting multiple clotting factors such as factors II, IX, X, XI, and XII, which depend on calcium for their activation. Blood coagulation factor XII (FXII) activates when it binds to artificial, negatively charged surfaces such as biomaterials. This activation transforms FXII into the enzyme factor XIIa, which activates FXI, leading to the generation of thrombin [1]. FXII activation can occur in vitro and has been shown to have a role in intravascular thrombus formation, which makes it ideal for our research.
The surfaces of vascular biomaterials interact with blood components, triggering coagulation through the intrinsic clotting cascade. To reduce the risk of thrombogenesis, anticoagulants such as sodium citrate, sodium ethylenediaminetetraacetic acid (EDTA), and sodium heparin are commonly employed. These anticoagulants chelate calcium ions, thereby lowering the concentration of free calcium in the blood and inhibiting calcium-dependent clotting factors, including factors II, IX, X, XI, and XII. Blood coagulation factor XII (FXII) is particularly relevant, as it is activated upon binding to artificial, negatively charged surfaces, such as those of vascular stents or grafts, leading to the generation of thrombin [1]. In addition to FXII activation, soluble fibrinogen is converted into insoluble fibrin during thrombin generation. This conversion can be detected through changes in light absorbance within a blood plasma sample. The formation of fibrin also promotes platelet aggregation, further contributing to the development of a hemostatic clot [2].
This protocol monitors the production of fibrin by measuring the change in opacity of PPP or PRP over time, using the absorbance measured at a wavelength of 405 nm. This method provides insights into the thrombogenicity of various biomaterials and evaluates the influence of biological factors such as anticoagulants on blood plasma.
The use of platelet-rich plasma (PRP) vs. platelet-poor plasma (PPP) relies on the type of information being tested. PRP has a higher-than-normal amount of platelets, whereas PPP has barely any platelets at all. PRP contains several growth factors that support tissue regeneration, such as epidermal growth factor (EGF), transforming growth factor-beta (TGF-b), platelet-derived growth factor (PDGF), and insulin-like growth factor 1 (IGF-1) [3]. PRP also contains more calcium-binding proteins and clotting factors, whereas PPP contains more fibrinogen and lacks platelet-specific components. PRP may be more helpful in regenerative studies, while PPP may be useful in studies where platelets complicate the analysis of results. PRP clots faster than PRP due to the presence of platelets.
Sodium citrate, EDTA, and heparin are all capable of chelating calcium ions; however, they are not equally effective in this method. While heparin inhibits blood coagulation factor IV (calcium ions), it also inhibits other clotting factors that will not be reactivated by the recalcification of blood. As such, we expect this protocol to be less effective when using heparin vs. other anticoagulants. Other similar methods [4] include a partial thromboplastin time (PTT) test, which is used clinically to evaluate blood disorders based on recalcifying blood and measuring the time it takes to clot. Our protocol can be used for clinical applications as well; however, it is targeted toward the evaluation and improvement of biomaterials. Because it evaluates various anticoagulants for their efficacy in creating a thrombus after recalcification, our protocol could also be used to evaluate the efficacy of various anticoagulants in calcium chelation. In addition, this protocol can be used to evaluate the activation of FXII on various biomaterials.
Materials and reagents
Biological materials
1. Pooled human plasma (blood-derived, anticoagulant: Na heparin) (Innovative Research)
2. Pooled human plasma (blood-derived, anticoagulant: Na citrate) (Innovative Research)
3. Pooled human plasma (blood-derived, anticoagulant: Na EDTA) (Innovative Research)
4. Whole donor blood (anticoagulant: Na Citrate) (Zen-Bio)
5. Collagen from rat tail, Bornstein and Traub Type 1, powder (Sigma-Aldrich)
Reagents
1. Calcium chloride dihydrate, 99%, for analysis by the American Chemical Society (ACS) (Acros Organics, catalog number: 423525000)
2. 2× (50 mM) 4-(2-hydroxylethyl)-1-piperazineethanesulfonic acid (HEPES) buffered saline, diluted to 1× (25 mM) (Boston Bioproducts, catalog number: BM-165-250ml), storage temperature 4 °C
3. Ethanol (50%, 75%, 95%, 100%) diluted with DI water
4. Dulbecco’s phosphate-buffered saline (PBS) 10×, diluted to 1× (Corning, catalog number: 20-031-CV)
5. Deionized (DI) or reverse osmosis (RO) water
6. 0.02 N acetic acid (Fischer Scientific, catalog number: LC103902)
7. Heprasil, 1% wt (Biomatrix, catalog number: GS215)
8. Heparin thiol, MW 27K (Creative PEG Works, catalog number: HP-211)
9. Glutaraldehyde, 25%, diluted to 2.5% (Fischer Scientific, catalog number: O2957-1)
Biomaterials
1. Medtronic Integrity bare metal coronary stent (4 mm × 15 mm)*
2. Medtronic Zotarolimus eluting coronary stent (4 mm × 15 mm)*
3. Abbott Graftmaster coronary stent graft (4 mm × 15 mm)*
4. GORE® Viabahn 4–6 mm vascular graft (Gore Medical)*
5. Coverslips mini 5 mm diameter (World Precision Instruments)
*Note: Any length works, but 4 mm diameter tubular implants fit 96-well plates the best.
Solutions
1. 25 mM calcium chloride (see Recipes)
2. Collagen coating solution (see Recipes)
3. 1% heparin thiol (see Recipes)
Recipes
1. 25 mM calcium chloride
| Reagent | Final concentration | Amount |
|---|---|---|
| Calcium chloride dihydrate | 25 mM | 13.4 mg |
| DI/RO H2O | n/a | 5 mL |
| Total | 25 mM | 5 mL |
2. Collagen coating solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Collagen from rat tail | 50 μg/mL | 1.5 mg |
| Acetic acid, 0.02 N | 0.02 N | 30 mL |
| Total | n/a | 30 mL |
3. 1% heparin thiol solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Heparin thiol, MW 27K | 1% | 0.1 mL |
| DI/RO H2O | n/a | 2.6 mL |
| Total | n/a | 2.7 mL |
Laboratory supplies
1. Low adhesion 96-well plate (SPL 3DTM Cell Floater Plate, 96 well, flat bottom, catalog number: 39796)
2. Eppendorf tubes (Flex Tube, catalog number: 022364120)
3. Tweezers (from any commercial source)
4. Stereomicroscope (Amscope, 10× magnification); any low-magnification microscope will work
5. Wire cutters (Xuron 410 Micro-shear); any sharp shear wire cutters will work
6. Warm water bath (bucket, warm water, thermometer to check temperature) or water bath (see Equipment)
7. Glass jar round vacuum desiccator (any brand)
Equipment
1. Flat plate orbital shaker (Lab Fish, model: OS-20F)
2. Centrifuge (Eppendorf, model: 5804)
3. Plate reader (Molecular Devices, model: SpectraMax iD3)
4. Scanning electron microscope (JEOL, model: JSM 6480LV)
5. Pipette and pipette tips, 20–200 μL and 200–1,000 μL (Eppendorf Pipettemaster); a multi-pipette is recommended but not required
6. Water bath (Fisher Scientific, model: Isotemp Digital Water Bath)
7. Stryker inflator with stopcock
8. UV cross-linker (OmniCure, model: Series 1500)
Software and datasets
1. Excel (Version 16.7); requires license: Microsoft 365 subscription
2. MATLAB (version R2023b, 11/28/2023); other graphics software, such as Python and matplotlib, can be used instead
Procedure
文章信息
稿件历史记录
提交日期: Dec 20, 2024
接收日期: Apr 8, 2025
在线发布日期: May 8, 2025
出版日期: May 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Sallee, A., Battistella, A., Johnson, R., Duong, A., Tan, W. and Keeling, N. M. (2025). Quantifying Thrombogenicity: A Bioanalytical Protocol for the Absorbance-Based Assessment of Vascular Implants with Plasma. Bio-protocol 15(10): e5316. DOI: 10.21769/BioProtoc.5316.
分类
医学 > 心血管疾病
生物工程 > 生物医学工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










