- EN - English
- CN - 中文
Dissection and Whole-Mount Immunofluorescent Staining of Mouse Hind Paw Muscles for Neuromuscular Junction Analysis
小鼠后足肌肉的解剖与整体免疫荧光染色:用于神经肌肉接头分析
发布: 2025年05月20日第15卷第10期 DOI: 10.21769/BioProtoc.5315 浏览次数: 3943
评审: Helena ChaytowMarion HoggAnonymous reviewer(s)
Abstract
The neuromuscular junction (NMJ) is a peripheral synaptic connection between a lower motor neuron and skeletal muscle fibre that enables muscle contraction in response to neuronal stimulation. NMJ dysfunction and morphological abnormalities are commonly observed in neurological conditions, including amyotrophic lateral sclerosis, Charcot–Marie–Tooth disease, and spinal muscular atrophy. Employing precise and reproducible techniques to visualise NMJs in mouse models of neuromuscular disorders is crucial for uncovering aspects of neuropathology, revealing disease mechanisms, and evaluating therapeutic approaches. Here, we present a method for dissecting the deep lumbrical and flexor digitorum brevis (FDB) muscles of the mouse hind paw and describe the process of whole-mount immunofluorescent staining for morphological analysis of NMJs. Similar whole-mount techniques have been applied to other muscles, such as the diaphragm; however, dense connective tissue in adult samples often impedes antibody penetration. Moreover, large hind limb muscles, including the gastrocnemius and tibialis anterior, are commonly used to examine NMJs but require embedding and cryosectioning. These additional steps increase the complexity and duration of the protocol and can introduce sectioning artefacts, including transection of NMJs and disruption of morphology. Using small hind paw muscles enables whole-mounting, which completely eliminates the requirement for embedding and cryosectioning. As a result, the entire neuromuscular innervation pattern can be visualised, allowing a more accurate assessment of NMJ development, denervation, and regeneration in mouse models of neurological disease and nerve injury, which can be applied across all postnatal ages.
Key features
• Small muscles of the mouse hind paw, i.e., lumbrical and FDB muscles, can be rapidly dissected for whole-mount immunofluorescent analysis without the need for cryosectioning.
• This protocol allows visualisation of the entire neuromuscular innervation pattern using axonal (anti-tubulin βIII), pre-synaptic (anti-synaptophysin), and post-synaptic (α-bungarotoxin) markers.
• Whole-mount immunofluorescence of hind paw muscles enables assessment of developmental, degenerative, and regenerative phenotypes in young and adult mice across disease and injury models.
• High-throughput analysis can be performed using NMJ-Analyser or NMJ-morph to evaluate diverse morphological features of the NMJ.
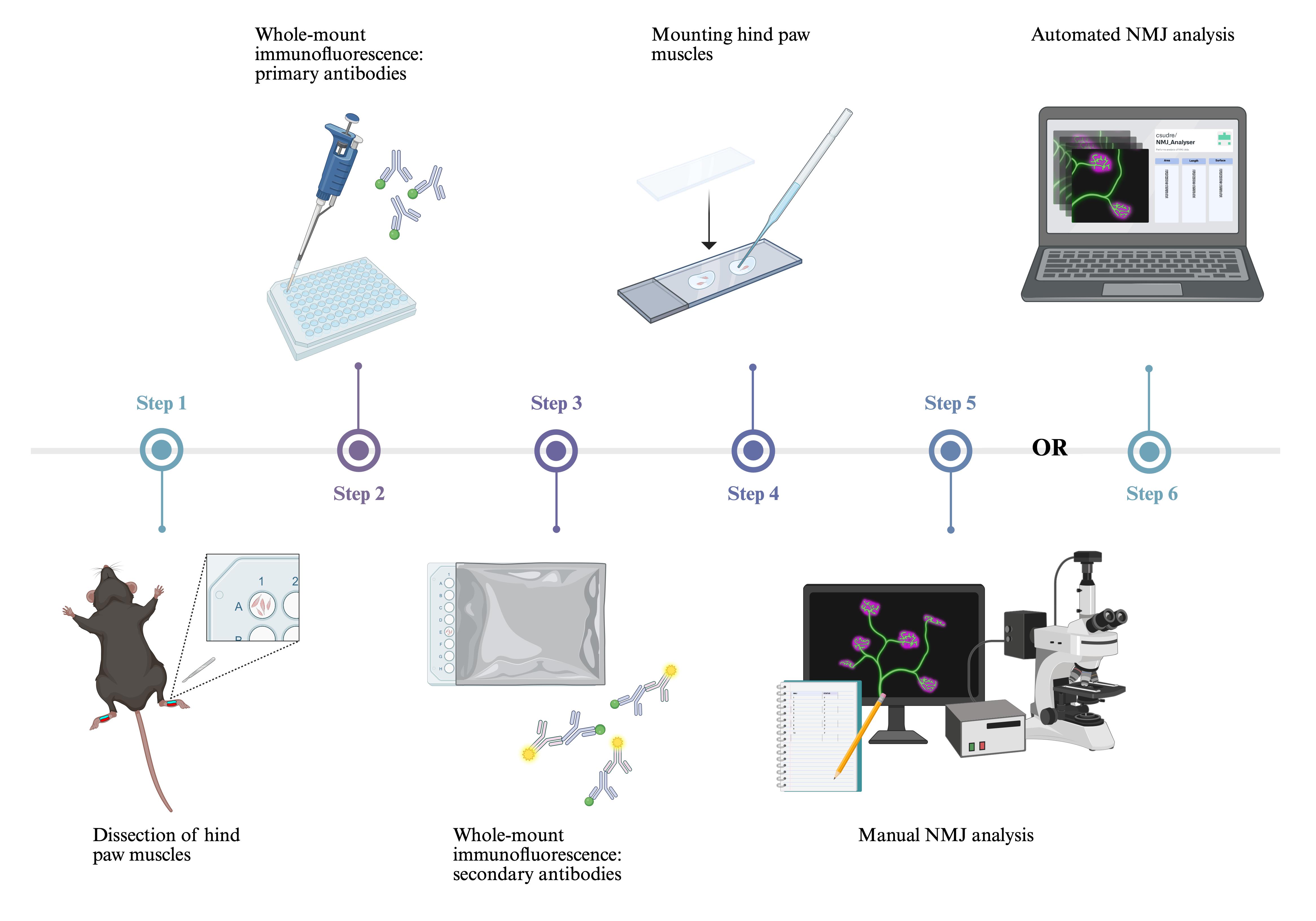
Keywords: α-bungarotoxin (αBTX) (α-银环蛇毒素(αBTX))Graphical overview
Background
The neuromuscular junction (NMJ) is a specialised synapse connecting the axon terminal of a lower motor neuron to a skeletal muscle fibre. To generate muscle contraction, the pre-synaptic motor terminal releases acetylcholine in response to an action potential. Acetylcholine traverses the synaptic cleft and binds to acetylcholine receptors (AChRs) clustered in the post-synaptic muscle membrane [1]. Due to its experimental accessibility and well-defined structure, the NMJ serves as a valuable model for studying synaptic formation, organisation, maturation, and function [2,3].
NMJ dysfunction is a principal feature of numerous neurological disorders, as observed in patients and mouse models of amyotrophic lateral sclerosis (ALS) [4], Charcot–Marie–Tooth disease (CMT) [5], and spinal muscular atrophy (SMA) [6,7]. Moreover, the NMJ is the primary site of pathology in congenital myasthenic syndromes [8] and several autoimmune diseases, including myasthenia gravis, in which autoantibodies target post-synaptic AChRs [9]. Muscular dystrophies [10], ageing [11], and toxic neuropathies, such as those induced by chemotherapeutic agents [12], also result in structural and/or functional abnormalities at the NMJ, contributing to muscle weakness and motor dysfunction.
Large muscles of the hind limb, such as the tibialis anterior, soleus, and gastrocnemius, have been traditionally used for NMJ evaluation [13,14]. Distal muscles are valuable for investigating length-dependent diseases such as CMT, where bilateral weakness and sensory deficits mainly affect the extremities [15]. To image NMJs in these thicker muscles, teasing or cryosectioning is required to achieve sufficient antibody penetration. However, this process is time-consuming and can introduce artefacts from inconsistent fixation and sectioning. While whole-mount immunofluorescence of thinner muscles (e.g., diaphragm) addresses these limitations, dense connective tissue in adult samples can impede antibody penetration and thus restrict application to only embryonic and neonatal mice [16]. This poses challenges for studying neurological disease models, where analysis of adult tissues is critical for examining disease progression or late-stage pathology.
We present a method for whole-mount immunostaining of the deep lumbricals and flexor digitorum brevis (FDB). These fast-twitch, hind paw muscles exhibit significant NMJ denervation in mouse models of ALS, CMT, and SMA [7,17,18], underlining their relevance to studies of neuromuscular disease. Their small, thin structure eliminates the requirement for embedding and cryosectioning, thus reducing preparation time and minimising experimental artefacts. NMJs are smaller than the usual section thickness of 30–50 μm, extending <15 μm along the z-dimension [14]. As a result, cryosectioning of larger muscles often transects NMJs and prevents visualisation of the entire innervation pattern. Moreover, since NMJs are not always aligned along the length of the muscle and can span a variety of orientations in three-dimensional space, cryosectioning often leads to fragmented or incomplete visualisation of endplates, compromising their spatial arrangement. In contrast, our approach enables three-dimensional confocal imaging of the complete neuromuscular network within a manageable z-stack depth, providing high-resolution, comprehensive, and accurate analysis of NMJ development and degeneration [19]. Importantly, the method is appropriate for assessing both young and adult mice.
Our protocol offers broad applicability for investigating several aspects of NMJ biology, e.g., endplate size [20], innervation status [21], nodal sprouting [22], AChR turnover [23,24], and axonal regeneration following injury [25]. The use of additional antibodies further extends the utility of this protocol. For instance, terminal Schwann cells (tSCs) can be identified using anti-S100, and their growth and morphology can be assessed, with a significant reduction in tSC sprouting serving as evidence for impaired remodelling capacity [26]. These applications establish this method as a powerful approach for exploring NMJ function and pathology.
Materials and reagents
Note: Similar materials, reagents, and equipment can be purchased from alternative sources.
Biological materials
1. Mouse
Note: This protocol is suitable for mice of any post-natal age, sex or genetic background. Mice can be sourced from any reputable supplier, such as Charles River or The Jackson Laboratory (JAX).
Reagents
Dissection of hind paw muscles
1. 16% paraformaldehyde (PFA) (Thermo Scientific, catalog number: 28908)
2. Milli-Q® water
3. OxoidTM phosphate buffered saline tablets (PBS) (Thermo Scientific, catalog number: BR0014G)
4. Sodium azide (Sigma-Aldrich, catalog number: S2002)
5. Sodium pentobarbital (Merial Animal Health Ltd., catalog number: QN51AA01)
6. Ethanol absolute ≥99.8% (VWR, catalog number: 20821.300)
Whole-mount muscle immunohistochemistry
1. Alexa Fluor 488 goat anti-guinea pig (H+L) antibody (Invitrogen, catalog number: A11073, RRID: AB_2534117)
2. Alexa Fluor 555 conjugated α-bungarotoxin (Invitrogen, catalog number: B35451)
3. Anti-synaptophysin (Nittobo, catalog number: MSFR105690, RRID: AB_2571843)
4. Anti-tubulin βIII (Nittobo, catalog number: MSFR105990, RRID: AB_2571849)
5. Bovine serum albumin (BSA) fraction V (Roche, catalog number: 10735094001)
6. Fluoromount-G mounting medium (Invitrogen, catalog number: 00-4958-02)
7. Milli-Q® water
8. OxoidTM PBS tablets (Thermo Scientific, catalog number: BR0014G)
9. Triton X-100 (Sigma, catalog number: T8787)
Solutions
1. 1× PBS (see Recipes)
2. 10× PBS (see Recipes)
3. 4% (w/v) PFA in 1× PBS (see Recipes)
4. 70% (v/v) ethanol in Milli-Q® water (see Recipes)
5. 2% (v/v) Triton X-100 in 1× PBS (see Recipes)
6. 2% (w/v) sodium azide in 1× PBS (see Recipes)
7. 0.02% (w/v) sodium azide in 1× PBS (see Recipes)
8. Blocking solution [4% (w/v) BSA and 1% (w/v) Triton X-100 in 1× PBS] (see Recipes)
Recipes
1. 1× PBS (1 L)
Note: Add reagents to a 1 L glass bottle and place on a magnetic stirrer until tablets have dissolved. Store at room temperature (RT) (18–22 °C) for up to six months.
| Reagent | Final concentration | Final quantity or volume |
|---|---|---|
| OxoidTM PBS tablets | 137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4 | 10 tablets |
| Milli-Q® | n/a | 1 L |
2. 10× PBS (100 mL)
Note: Add reagents to a 100 mL glass bottle and place on a magnetic stirrer until tablets have dissolved. Store at RT. This solution can be stable for many months, provided it remains non-contaminated.
| Reagent | Final concentration | Final quantity or volume |
|---|---|---|
| OxoidTM PBS tablets | 1.37 M NaCl, 30 mM KCl, 80 mM Na2HPO4, 15 mM KH2PO4 | 10 tablets |
| Milli-Q® | n/a | 100 mL |
3. 4% (w/v) PFA in 1× PBS (40 mL)
Note: For best results, the solution should be freshly prepared before use. Store the solution in a 50 mL centrifuge tube. Unused aliquots can be maintained for one week at 4 °C or several months at -20 °C.
| Reagent | Final concentration | Final quantity or volume |
|---|---|---|
| 16% PFA | 4% | 10 mL |
| 10× PBS | 1× | 4 mL |
| Milli-Q® | n/a | 26 mL |
4. 70% (v/v) ethanol in Milli-Q®water (50 mL)
Note: Store at RT for up to one month. Prepare the solution in a 50 mL centrifuge tube.
| Reagent | Final concentration | Final quantity or volume |
|---|---|---|
| Ethanol (≥99.8%) | 70% | 35 mL |
| Milli-Q® | n/a | 15 mL |
5. 2% (v/v) Triton X-100 in 1× PBS (40 mL)
Note: Store at 4 °C for a maximum of three months. Prepare the solution in a 50 mL centrifuge tube. Keep away from light to prevent degradation.
| Reagent | Final concentration | Final quantity or volume |
|---|---|---|
| Triton X-100 | 2% | 0.8 mL |
| 1× PBS | 1× | 39.2 mL |
6. 2% (w/v) sodium azide in 1× PBS (50 mL)
Note: Store at 4 °C for up to 12 months. Sodium azide is toxic; ensure the use of appropriate personal protective equipment and follow all relevant safety protocols when handling it.
| Reagent | Final concentration | Final quantity or volume |
|---|---|---|
| Sodium azide | 2% | 1 g |
| 1× PBS | 1× | 50 mL |
7. 0.02% (w/v) sodium azide in 1× PBS (10 mL)
Note: Store at 4 °C for up to 12 months. Sodium azide is toxic; ensure the use of appropriate personal protective equipment and follow all relevant safety protocols when handling it.
| Reagent | Final concentration | Final quantity or volume |
|---|---|---|
| 2% sodium azide | 0.02% | 0.1 mL |
| 1× PBS | 1× | 9.9 mL |
8. Blocking solution [4% (w/v) BSA and 1% (w/v) Triton X-100 in 1× PBS] (10 mL)
Note: Store at 4 °C and use within one week.
| Reagent | Final concentration | Final quantity or volume |
|---|---|---|
| BSA | 4% | 0.4 g |
| 2% Triton X-100 | 1% | 5 mL |
| 1× PBS | 1× | 5 mL |
Laboratory supplies
Dissection of hind paw muscles
1. 1 L glass bottle (VWR, catalog number: 215-1595)
2. 100 mL glass bottle (VWR, catalog number: 215-1592)
3. 12 mm × 0.2 mm minutiens Austerlitz Insect Pins® (Entomoravia, catalog number: 0.20)
4. 50 mL centrifuge tubes (VWR, catalog number: 734-0448)
5. 60 mm × 15 mm Petri dishes (BD Biosciences, catalog number: 351007)
6. 96-well CytoOne TC-treated plate (Starlab, catalog number: CC7682-7596)
7. BD MicrolanceTM 3 hypodermic needle 30 G × 0.5” (Fisher Scientific, catalog number: 10442014)
8. BD PlastiPak 1 mL syringe (Fisher Scientific, catalog number: 15489199)
9. Bone scissors (Fine Science Tools, catalog number: 14110-15)
10. Curved forceps (Dumont Swissmade, catalog number: 0102-7-PO)
11. Magnetic stirring bar (VWR, catalog number: 442-0272)
12. Small spring scissors (Deutsche BioMedical, catalog number: DBV1005)
13. Straight forceps (Dumont Swissmade, catalog number: 0208-5-PO)
14. SYLGARD® 184 silicone elastomer kit (Scientific Laboratory Supplies, catalog number: 63416.5S)
Note: Prepare silicone-coated Petri dishes at least one day prior to dissection. Mix 100 g of polydimethylsiloxane SYLGARD® 184 with 10 g of curing agent. Pour the mixture into 60 mm × 15 mm Petri dishes and cure overnight at RT.
Whole-mount muscle immunohistochemistry
1. 1.5 mL TubeOne® microcentrifuge tubes (Starlab, catalog number: S1615-5500)
2. 200 m × 326 mm aluminium foil (Scientific Laboratory Supplies, catalog number: 39400)
3. 22 × 64 × 0.13–16 mm cover glass (VWR, catalog number: 631-0880)
4. 3 mL plastic transfer pipettes (Scientific Laboratory Supplies, catalog number: PIP4210)
5. 50 mL centrifuge tubes (VWR, catalog number: 734-0448)
6. 96-well CytoOne TC-treated plate (Starlab, catalog number: CC7682-7596)
7. Polysine®-coated slides (VWR, catalog number: 631-0107)
8. Slide box (Scientific Laboratory Supplies, catalog number: MIC3550)
9. Straight forceps (Dumont Swissmade, catalog number: 0208-5-PO)
10. Kimtech ScienceTM delicate task wipes (Fisher Scientific, catalog number: 15402680)
Equipment
Dissection of hind paw muscles
1. Magnetic stirrer (VWR, catalog number: 422-0883)
2. Dissection microscope, e.g., Motic DSK-500 dual observation stereomicroscope (Motic, catalog number: PM5539B901)
Whole-mount muscle immunohistochemistry
1. Dissection microscope, e.g., Motic DSK-500 dual observation stereomicroscope (Motic, catalog number: PM5539B901)
2. Rotamax 120 shaker (Heidolph, catalog number: 544-41200-00)
Imaging
1. Confocal microscope (Zeiss, model: LSM 780)
Note: Any confocal microscope that provides high-resolution images suitable for automated NMJ scoring should suffice.
Software and datasets
1. ImageJ (Version 2.14.0/1.54f, 2023; https://imagej.net/software/fiji/)
2. NMJ-Analyser [21] or NMJ-morph [27]
Note: These references provide detailed instructions on how to install and use NMJ-Analyser or NMJ-morph.
Procedure
文章信息
稿件历史记录
提交日期: Jan 30, 2025
接收日期: Apr 18, 2025
在线发布日期: May 5, 2025
出版日期: May 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Simkin, R. L., Rhymes, E. R., Lang, Q., Birsa, N. and Sleigh, J. N. (2025). Dissection and Whole-Mount Immunofluorescent Staining of Mouse Hind Paw Muscles for Neuromuscular Junction Analysis. Bio-protocol 15(10): e5315. DOI: 10.21769/BioProtoc.5315.
分类
神经科学 > 神经解剖学和神经环路
神经科学 > 周围神经系统
细胞生物学 > 组织分析 > 组织染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link