- EN - English
- CN - 中文
Tracking Oral Nanoparticle Uptake in Mouse Gastrointestinal Tract by Fluorescent Labeling and t-SNE Flow Cytometry
小鼠胃肠道口服纳米颗粒摄取的荧光标记与t-SNE流式细胞术追踪研究
发布: 2025年05月20日第15卷第10期 DOI: 10.21769/BioProtoc.5309 浏览次数: 3025
评审: Komuraiah MyakalaKanchan BhasinHsih-Yin Tan

相关实验方案

外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1448 阅读
Abstract
The growing demand for advanced analytical techniques to explore complex cellular targets of nanotherapeutics has driven the development of innovative methodologies. This protocol presents a refined approach for fluorescent labeling and flow cytometric analysis of colonic cells following oral lipid nanoparticle (LNP) treatment, focusing on LNP uptake in colonic cell subpopulations in a DSS-induced colitis mouse model. By integrating optimized fluorochrome selection and gating strategies with advanced t-distributed stochastic neighbor embedding (t-SNE) analysis, this method enables precise identification and multidimensional visualization of LNP-targeted epithelial and macrophage populations under the complex conditions of inflamed colon tissue. Building on our previous studies demonstrating the effectiveness of nanoparticles in targeted drug delivery, this approach highlights the utility of flow cytometry for assessing uptake efficiency and cellular targeting. Unlike conventional protocols, it incorporates t-SNE for enhanced multidimensional analysis, allowing for the detection of subtle cellular patterns and the delineation of intricate clusters. By addressing gaps in traditional methodologies, this protocol provides a robust and reproducible framework for investigating in vivo cellular targets and optimizing drug delivery strategies for nanomedicines.
Key features
• This protocol is optimized for investigating nanoparticle uptake in inflamed colonic tissues from DSS-induced colitis models.
• This protocol integrates flow cytometry with t-SNE for high-dimensional data analysis, enabling detailed characterization of cellular populations.
Keywords: Oral lipid nanoparticles (口服脂质纳米颗粒)Graphical overview
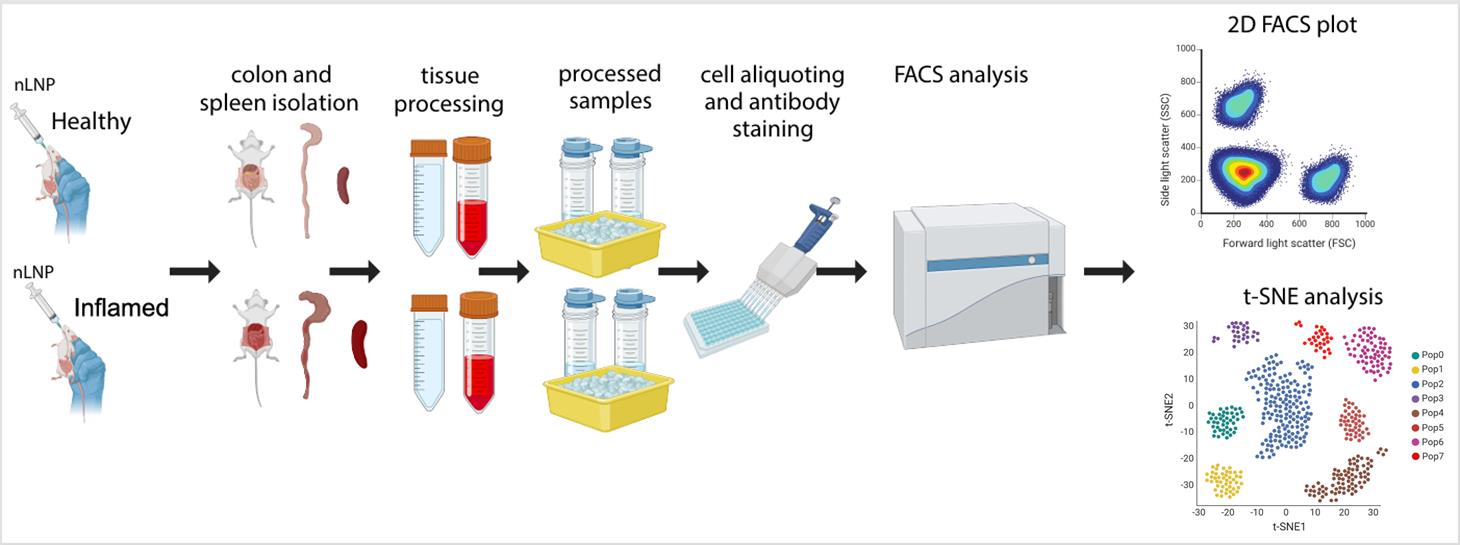
Evaluation of new lipid nanoparticle (nLNP) uptake in healthy and inflamed mouse colon using flow cytometry and t-SNE analysis. This schematic outlines the step-by-step procedure, including nLNP administration, tissue isolation and processing, antibody staining and protocol optimization, flow cytometry (FACS) data acquisition, and subsequent data analysis and validation. This image was created with BioRender.
Background
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease, is characterized by chronic inflammation of the gastrointestinal (GI) tract and poses significant therapeutic challenges [1,2]. Conventional treatments often suffer from limited efficacy and significant systemic side effects due to nonspecific drug biodistribution. Lipid nanoparticles (LNPs) have emerged as a promising drug delivery platform, offering targeted delivery capability and enhanced biocompatibility [3,4]. However, assessing their cellular targets and biodistribution in the GI tract, particularly under inflammatory conditions, remains a challenge.
Existing methods for studying in vivo nanoparticle uptake, such as basic flow cytometry, provide useful insights but lack the dimensionality needed to resolve complex cellular heterogeneity. Recent advances in data analysis techniques, particularly t-distributed stochastic neighbor embedding (t-SNE), enable high-dimensional visualization and clustering, improving the identification of intricate cellular subpopulations [5,6].
This protocol builds upon previously established methods for studying nanoparticle–cell interactions by integrating t-SNE analysis into flow cytometric workflows [7]. This approach enables precise identification of colonic epithelial and macrophage populations in complex inflamed colon tissues and enhances the detection of subtle cellular patterns that traditional gating strategies may overlook. Unlike previous studies relying on single-dimensional gating, this protocol offers a robust multidimensional assessment of nanoparticle targeting and uptake.
Beyond its application to LNPs, this protocol can be adapted for other drug delivery systems, such as polymeric nanoparticles or exosomes, and in various disease models. Additionally, the integration of t-SNE into flow cytometry establishes a framework for studying cellular heterogeneity in other inflamed or complex tissues, such as respiratory epithelia. By addressing key limitations of traditional methodologies, this protocol advances nanoparticle drug delivery research and provides a powerful tool for exploring in vivo targeting mechanisms of nanoparticles.
Materials and reagents
Biological materials
1. C57BL/6J mice (Jackson Laboratory, female, 6–7 weeks of age)
Reagents
1. DAPI (Thermo Fisher Scientific, catalog number: 62247)
2. PBS (Corning, catalog number: 21-040-CV)
3. FBS (R&D Systems, catalog number: S11150H)
4. Sodium azide (Teknova, catalog number: S0209)
5. LIVE/DEAD Fixable Near IR Viability kit (Thermo Fisher Scientific, catalog number: L34982)
6. HBSS (Cytiva, catalog number: SH3058801)
7. ACK lysing buffer (Thermo Fisher Scientific, catalog number A1049201)
8. RPMI (Thermo Fisher Scientific, catalog number: 11875119)
9. CD45 (Alexa Fluor 700, clone: 30-F11, dilution: 1:800) (Thermo Fisher Scientific, catalog number: 56-045180)
10. EpCAM (APC-eFluor 780, clone: G8.8, dilution: 1:300) (Thermo Fisher Scientific, catalog number: 47-579181)
11. Ly6C (BV 650, clone: HK1.4, dilution: 1:1,000) (BioLegend, catalog number: 128049)
12. CD11b (BV 780, clone: M1/70, dilution: 1:200) (BioLegend, catalog number: 101243)
13. F4/80 (APC, clone: BM8, dilution: 1:100) (Thermo Fisher Scientific, catalog number: 17-4801-82)
14. 2.4G2 (unconjugated, clone: 2.4G2, dilution: 1:100) (BioXcell, catalog number: BE0307)
15. Dextran sulfate sodium salt (DSS), colitis grade (molecular weight: 36,000–50,000 Da) (MP Biomedicals, catalog number: 0216011090)
16. EDTA (0.5 M, pH 8.0) (Invitrogen, catalog number: AM9260G)
17. Trypan blue, 0.4% (Thermo Fisher Scientific, catalog number: T10282)
18. TrypLE [+] phenol red (Gibco, Thermo Fisher Scientific, catalog number: 12605010)
Laboratory supplies
1. 96-well, round-bottom plates (Corning, catalog number: 3797)
2. Cell strainer, pore size 100 μm, sterile (Corning, catalog number: CLS352360)
3. Cell strainer, pore size 40 μm, sterile (Corning, catalog number: CLS431750)
Equipment
1. Cytoflex LX flow cytometer (Beckman Coulter, N3-V5-B3-Y5-R3-I2, 2-parameter system equipped with 6 lasers: 375, 405, 488, 561, 638, 808 nm)
2. Automated cell counter (Invitrogen, model: Countess 3 FL)
3. Centrifuge (Eppendorf, model: 5810R)
Software and datasets
1. FlowJo (v10.10.0; BD)
2. Prism (v10.1.2; GraphPad)
3. BioRender (https://www.biorender.com/)
Procedure
文章信息
稿件历史记录
提交日期: Feb 3, 2025
接收日期: Apr 8, 2025
在线发布日期: Apr 26, 2025
出版日期: May 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Mow, R. J., Kuczma, M. P., Shi, X., Merlin, D. and Yang, C. (2025). Tracking Oral Nanoparticle Uptake in Mouse Gastrointestinal Tract by Fluorescent Labeling and t-SNE Flow Cytometry. Bio-protocol 15(10): e5309. DOI: 10.21769/BioProtoc.5309.
分类
免疫学 > 免疫细胞分离 > 巨噬细胞
免疫学 > 炎症性疾病
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












