- EN - English
- CN - 中文
Standardized Flow Cytometry Method for Absolute Counting of Intraepithelial Lymphocytes in the Intestinal Mucosa Using TruCountTM Beads
基于TruCount™微珠的肠上皮内淋巴细胞绝对计数标准化流式细胞术方法
(*contributed equally to this work) 发布: 2025年05月05日第15卷第9期 DOI: 10.21769/BioProtoc.5307 浏览次数: 2196
评审: Pilar Villacampa AlcubierreMinal EngavaleAnonymous reviewer(s)
Abstract
In the intestinal epithelium, intraepithelial lymphocytes (IELs) coexist with intestinal epithelial cells (IECs). The IELs have an important role in defending the intestinal tract against pathogens and eliminating tumor cells. Anomalies in the absolute IEL count have been reported in various digestive diseases. IELs are typically counted using histologic techniques or under light microscopy after isolation of the epithelium. However, these techniques can introduce bias, which might account for the discrepancies in counts from one study to another. Here, we describe a flow cytometry assay for determining the absolute IEL count and the IEL/IEC ratio. We combined a conventional epithelial isolation method with a BD TruCountTM bead-based absolute counting technique to quantify IELs (CD45+ CD326/EpCAM- CD103+CD3+) and IECs (CD45- CD326/EpCAM+) in a C57BL/6 mouse model.
Key features
• Intraepithelial lymphocytes (IELs) play a crucial role in maintaining mucosal integrity and defending against pathogens.
• Conventional manual counting of IELs using a hemocytometer relies heavily on the operator's expertise.
• Flow cytometry offers a more standardized approach to cell counting.
• Using TruCountTM beads to quantify IELs and intraepithelial cells (IECs) by flow cytometry and assess their ratio ensures reproducibility and comparison with immunohistochemical methods.
Keywords: Intraepithelial lymphocytes (上皮内淋巴细胞)Graphical overview
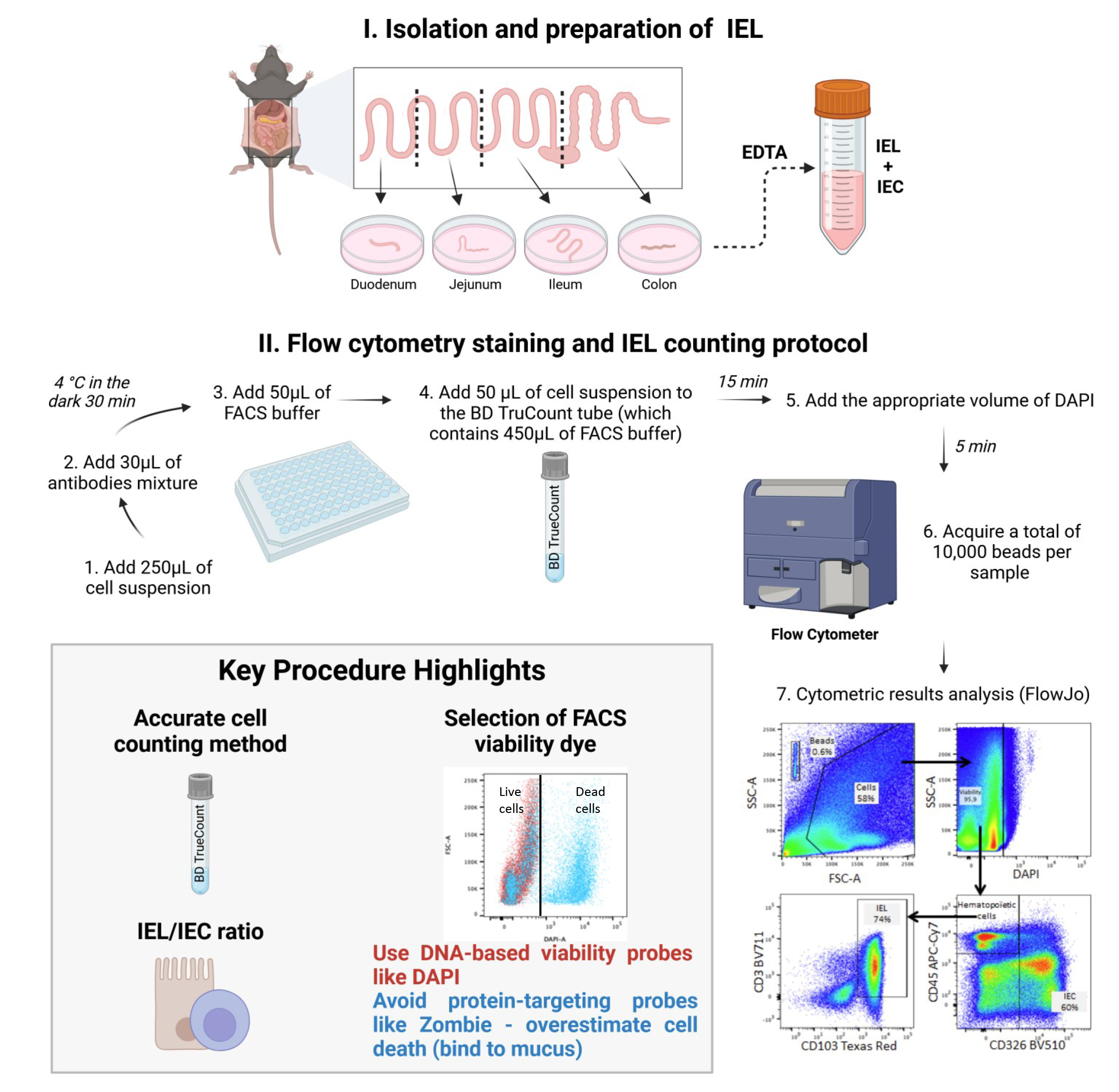
Background
Intestinal intraepithelial lymphocytes (IELs) are located between the epithelial cells of the digestive tract and can be divided into two main subpopulations: natural IELs and induced IELs. Natural IELs are mainly composed of γδT lymphocytes that develop and reside in the intestinal epithelium. In contrast, induced IELs are conventional CD4 and CD8 peripheral αβ T cells that migrate into the epithelium in response to antigens. Furthermore, small numbers of lymphocytes that do not express the T-cell receptor (TCR), such as intracytoplasmic (i)CD8 and iCD3 cells and type 1 innate lymphoid cells, are present in the epithelium.
IELs serve as the first line of defense against enteric pathogens and are vital for maintaining intestinal homeostasis [1]. Once activated, IELs are able to eliminate pathogens through the secretion of pro-inflammatory cytokines [e.g., interferon gamma (IFNγ)] and cytotoxic molecules (e.g., granzyme and perforin). These cells also have a regulatory role in the epithelium through the secretion of anti-inflammatory cytokines like interleukin (IL)-10 and transforming growth factor beta (TGF-β).
An imbalance in IEL inflammatory responses can significantly affect intestinal mucosa integrity. In celiac disease, for example, the unchecked expansion of IELs leads to the destruction of intestinal epithelial cells (IECs) and the atrophy of villi [2]. In patients with colorectal cancer, a greater number of TCRγδ IELs with a strong cytotoxic profile in the area around the tumor is associated with a lower risk of metastasis [3]. Although the specific role of this population in tumor cell regulation and elimination is not fully understood, one can hypothesize that deficiencies in this population substantially affect the patient’s response to treatments in general and to immunotherapy more specifically. Furthermore, many studies have documented anomalies in the total IEL count in various digestive pathologies [4].
Thus, an accurate assessment of the absolute IEL count is of great importance. A widely used approach for counting IELs involves analyzing histological sections. This multi-step process, including tissue fixation, paraffin embedding, block sectioning, slide mounting, and antibody labeling, extends experiment time compared to cytometric methods. Additionally, cell counting requires independent double readings by trained operators, similar to conventional hemocytometer counting of cell suspensions obtained after the epithelium has been detached from the rest of the mucosa, which also demands multiple readings by experienced personnel. So, a standardized procedure for counting IELs is lacking. Some experts express the IEL count with regard to the weight of the tissue, while others normalize it against the IEC count in the same sample. This lack of methodological consensus might explain the great disparities observed from one publication to another.
Recently, Lockhart et al. [5] employed flow cytometry to phenotype IELs and used BD TruCountTM beads to yield the absolute IEL count in murine intestinal mucosa. The high-precision BD TruCountTM procedure described below goes a step further by counting not only IELs but also IECs as an internal control in each sample, to ensure the assay’s reproducibility. Indeed, our flow cytometry technique is the first to have used BD TruCountTM beads to provide the absolute IEL account and the IEL/IEC ratio. The IEL population is phenotypically identified by the expression of CD3 and CD103, while the epithelial population is characterized by the expression of CD326/EpCAM+. Moreover, unlike cells of hematopoietic origin such as IELs, IECs do not express the CD45 marker. During the development of this protocol, we observed that protein-based viability probes, such as Zombie dye, resulted in an overestimation of the percentage of dead cells compared to nuclear viability probes like DAPI. Indeed, mucus that remains adherent to epithelial cells nonspecifically catches protein-based viability probes and is therefore responsible for off-target binding. This overestimation subsequently biased the IEL count when compared to conventional microscopic counting. This finding underscores the importance of carefully selecting the viability probe when using this type of assay. We believe that our assay protocol will help to advance research in this field by providing a new tool for studying IEL density in the intestinal epithelium and, potentially, in other mucosal tissues. Through its use of commercially available reagents and antibodies, this flow cytometry–based protocol offers the advantages of (i) standardization, ensuring reproducibility and, therefore, the reliability of results, (ii) time efficiency, as it reduces the overall duration of the process, (iii) reduced workforce requirements, as it minimizes the need for multiple operators, and (iv) improved practicality, as it simplifies implementation while maintaining accuracy. This method is applicable to all rodent models used in the laboratory, with the main limitation being the availability of suitable antibodies for cytometry, which may not exist for all species. The protocol can also be adapted for human samples, though adjustments in reagent quantities will be necessary due to variations in specimen size.
Materials and reagents
Biological materials
1. Female, 8-week-old C57BL/6 mice (Envigo, bred and maintained at the Faculté de Pharmacie de Paris, Université Paris Cité, Paris, France) (the results of 10 mice were compiled for the study)
Reagents
1. RPMI-GlutaMAX medium (Gibco, catalog number: 22400089)
2. HBSS (Gibco, catalog number: 14170088)
3. DPBS (Gibco, catalog number: 14190144)
4. Fetal bovine serum (FBS) (Eurobioscientific, catalog number: CVFSVF0601)
5. Gentamicin (Gibco, catalog number: 15710064)
6. Penicillin-streptomycin (Gibco, catalog number: 15140122)
7. DTT (Sigma-Aldrich, catalog number: D55455G)
8. EDTA (Invitrogen, catalog number: AM9260G)
9. BD TruCountTM tubes (BD Bioscience, catalog number: 48000)
10. Antibodies (see Table 1)
11. DAPI viability probe (Thermo Fischer, catalog number: D1306)
Caution: In this procedure, it is essential to use a viability probe that specifically binds to DNA, rather than protein-targeting probes like Zombie dye, to prevent overestimation of cell death.
Table 1. List and preparation of antibodies and reagents used in the staining protocol
| Name | Target | Fluoro-chrome | Clone | Isotype | Species | Reference | Supplier | Initial concentration (mg/mL) | Final concentration (μg/mL) | Dilution |
| Anti mouse CD103 PE/Dazzel 594 | CD103 | PE/Dazzel 594 | 2 E7 | IgG | Armenian Hamster | BLE121414 | Biolegend | 0.2 | 2 | 1/100 |
| Anti mouse CD45 APC Cy7 | CD45 | APC Cy7 | 30-F11 | IgG2b kappa | Rat | 103116 | Biolegend | 0.2 | 2 | 1/100 |
| Anti mouse Epcam Brillant Violet 510 | EpCam CD326 | BV510 | G8.8 | IgG2a kappa | Rat | 560596 | BD | 0.2 | 2 | 1/100 |
| Anti mouse CD3 Brillant Violet 711 | CD3 | BV711 | 17A2 | IgG2b kappa | Rat | BLE100241 | Biolegend | 0.2 | 2 | 1/100 |
| BD Trucount TM | PercP-Cy5.5 | 340334 | BD | |||||||
| DAPI | UV | D1306 | Thermo Fisher | 1 | 1 | 1/1,000 |
Solutions
1. FACS buffer (see Recipes)
2. Complete medium (see Recipes)
3. Solution 1 (S1) (see Recipes)
4. Solution 2 (S2) (see Recipes)
Recipes
1. FACS Buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DPBS | N/A | 500 mL |
| FBS | 10% | 50 mL |
| EDTA | 2 mM | 2 mL |
2. Complete medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| RPMI-GlutaMAX | N/A | 500 mL |
| FBS | 10% | 50 mL |
| Penicillin-streptomycin | 50 U/mL | 5 mL |
| Gentamicin | 0.05 g/L | 1 mL |
3. Solution 1 (S1)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| HBSS | N/A | 500 mL |
| FBS | 2.5% | 12.5 mL |
| Penicillin-streptomycin | 50 U/mL | 5 mL |
| Gentamicin | 0.05 g/L | 1 mL |
| DTT | 5 mM |
4. Solution 2 (S2)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| HBSS | N/A | 500 mL |
| FBS | 2.5% | 12.5 mL |
| Penicillin-streptomycin | 50 U/mL | 5 mL |
| Gentamicin | 0.05 g/L | 1 mL |
| EDTA | 5 mM | 5 mL |
Laboratory supplies
1. 50 mL Falcon® conical centrifuge tubes (Corning, catalog number: 352070)
2. 96-well plates, V-bottom (Thermo ScientificTM, catalog number: 10192161)
3. 70 μm filters (Corning, catalog number: 22363548)
4. 100 μm filters (Corning, catalog number: 22363549)
Equipment
1. Surgical instruments (curved forceps, forceps, flat scissors, curved scissors, and scalpel)
2. 30 cm ruler
3. Scale (Ohaus Corporation, model: E12130)
4. Heated shaker for 50 mL tubes (VWR® Professional 3500, model: Orbital Shaker Incubator)
5. Benchtop vortexer (JANKE KUNKEL VF2)
6. Refrigerated Eppendorf centrifuge (Centrifuge, model: 5810R)
7. Microbiological safety station (Thermo Scientific, model: microbiological safety cabinet type 2 category 2)
8. BD Fortessa Cytometer (BD, model: LSRFortessa LSRII) equipped with the following 5 lasers:
a. UV laser (355 nm) (used for markers such as DAPI)
b. Violet laser (405 nm) (for the Abs CD3 Brilliant Violet 711 and CD326 Brilliant Violet 510)
c. Blue laser (488 nm) (for the BD TruCountTM beads)
d. Green laser (561 nm) (for the Abs CD103 PE/Dazzle 594)
e. Red laser (640 nm) (for the Abs CD45-APC-Cy7)
The configuration of the BD Fortessa Cytometer is shown in Table 2. Any other cytometer with the same configuration and characteristics can be used for our protocol.
Table 2. BD LSR Fortessa Cytometer optical configuration
| LASER | Detectors | Fluorochromes |
| BLUE LASER (488 nm) | Blue detector 515–545 | Alexa Fluor 488; FITC; GFP; CFSE; YFP |
| Blue detector 685–735 | PerCP; PerCP-Cy5.5 | |
| RED LASER (640 nm) | Red detector 663–677 | APC; Cy5; Alexa Fluor 647; eFluor 660 |
| Red detector 708–752 | Alexa 700; APC-Alexa 700 | |
| Red detector 750–810 | APC-Cy7; APC-H7 | |
| VIOLET LASER (405 nm) | Violet detector 430–470 | Cascade Blue; Pacific Blue; Alexa 430; V450; BV421 |
| Violet detector 500–550 | AmCyan; Pacific Orange; BV510; Qdot 525 | |
| Violet detector 600–620 | Qdot 605; BV 605 | |
| Violet detector 650–670 | Qdot 655; eFluor 650; BV 650 | |
| Violet detector 685–735 | BV 711 | |
| Violet detector 750–810 | Qdot 800; BV 786 | |
| GREEN LASER (561 nm) | Green detector 578–593 | PE; Cy3; Tomato |
| Green detector 600–620 | PE-Texas Red; PI; ECD; mCherry; RFP | |
| Green detector 655–685 | PE-Cy5; 7-AAD | |
| Green detector 685–735 | PE-Alexa 700; PE-Cy5.5 | |
| Green detector 750–800 | PE-Cy7; PE-Alexa 750 | |
| ULTRA VIOLET LASER (355 nm) | Ultra Violet detector 425–475 | DAPI; Hoechst Blue; Indo-1 (violet) |
| Ultra Violet detector 515–545 | Indo-1 (blue) |
Please see Supplementary materials and refer to the following publication for details related to flow cytometry: Flow cytometry sample acquisition using a BD LSR Fortessa flow cytometer running FACSDiva (DOI: 10.21769/BioProtoc.4679).
Software and datasets
1. FACSDiva (BD, version 6.1.3)
2. FlowJo (FlowJo, version 10.10.0)
Procedure
文章信息
稿件历史记录
提交日期: Jan 29, 2025
接收日期: Apr 8, 2025
在线发布日期: Apr 29, 2025
出版日期: May 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Joulain, C., Bessoles, S., Chiron, A. S., Sarrabayrouse, G. and Hacein-Bey-Abina, S. (2025). Standardized Flow Cytometry Method for Absolute Counting of Intraepithelial Lymphocytes in the Intestinal Mucosa Using TruCountTM Beads. Bio-protocol 15(9): e5307. DOI: 10.21769/BioProtoc.5307.
分类
免疫学 > 粘膜免疫学 > 上皮
细胞生物学 > 细胞染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












