- EN - English
- CN - 中文
From Colonization to High Production and Plasmodium vivax Infection of Anopheles darlingi and Anopheles deaneorum: a Platform for Malaria Research
从种群建立到高效繁育及感染间日疟原虫:达林按蚊与迪恩按蚊的疟疾研究平台
发布: 2025年05月05日第15卷第9期 DOI: 10.21769/BioProtoc.5302 浏览次数: 1744
评审: Kirsten A. CoprenDavid PaulAnonymous reviewer(s)
Abstract
The mass rearing of anopheline mosquitoes under laboratory conditions is essential for advancing malaria research. It facilitates in-depth studies on mosquito biology, behavior, and genetics and their role in Plasmodium transmission. However, the colonization of Neotropical anophelines such as Anopheles darlingi—a primary malaria vector in the Amazon region—has proven particularly challenging due to its unique reproductive characteristics. Unlike other species that can initially be colonized using forced copulation methods and later adapt to natural mating, An. darlingi does not copulate under forced conditions. Recent breakthroughs in An. darlingi colonization have been achieved using flashlight induction techniques, which have enabled the establishment and maintenance of stable laboratory populations. These advancements have created new opportunities for vector control studies in Brazil, including the testing of innovative control methods and Plasmodium transmission-blocking strategies. This protocol offers a comprehensive, step-by-step guide for initiating and scaling up large laboratory colonies of An. darlingi and An. deaneorum, a secondary malaria vector. It details methods for copulation induction, colony management, and successful artificial infection of mosquitoes with Plasmodium vivax. The guide serves as a critical resource for establishing new Neotropical anopheline colonies from different populations, contributing to future malaria research and control efforts in the Amazon. Additionally, the establishment of Brazil’s first Malaria Vector Production and Infection Platform (Plataforma de Produção e Infecção de Vetores da Malária, PIVEM) has further supported the development of new control technologies and the study of P. vivax–Anopheles interaction, advancing efforts to combat malaria in the region.
Key features
• High production and experimental infection of Anopheles by Plasmodium vivax.
Keywords: Colony establishment (种群建立)Graphical overview
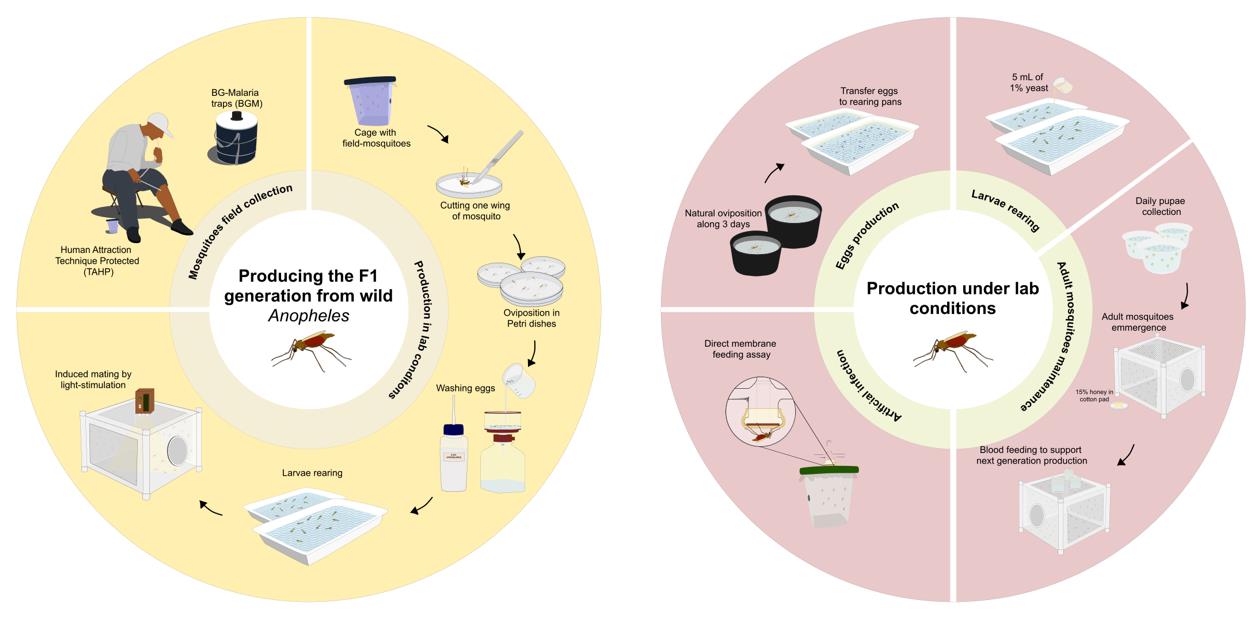
Graphical overview of laboratory procedure for F1 generation and maintenance of Anopheles darlingi and Anopheles deaneorum colonies. The procedure for producing the F1 generation begins with the capture of wild mosquitoes using the human attraction technique protected (TAHP) and BG-malaria traps (BGM). Captured females are induced to oviposit in Petri dishes following the first gonotrophic cycle under lab conditions. Egg hatching is stimulated by washing the eggs with a 0.25% hypochlorite solution. The larvae are reared to the pupae stage, after which the pupae are transferred to large cages until adults emerge. Once the laboratory colony achieves natural oviposition and mating, the maintenance procedures are streamlined to focus on egg production, larval rearing, and adult mating. Experimental infections with Plasmodium vivax parasites are performed using direct membrane feeding assays (DMFA).
Background
The mass rearing of Anopheles mosquitoes in laboratories is essential for malaria research, enabling controlled studies on their biology, behavior, and vector competence. While Anopheles gambiae sensu stricto (s.s.) has been extensively studied since its colonization in 1953 [1], key aspects of Anopheles darlingi (Root, 1926), the primary malaria vector in the Amazon region [2,3], remain poorly understood due to the absence, until recently, of sustainable laboratory colonies.
In 1990, an Anopheles deaneorum (Rosa-Freitas 1989) colony was first established using the force mating technique [4]; no other colony was successfully maintained until 2020 [5]. This species serves as a secondary vector in certain Amazonian regions and has the potential to expand its geographic range due to climate change [6,7].
Historically, establishing An. darlingi colonies under laboratory conditions has proven challenging. Attempts to establish An. darlingi colonies date back to 1947, with colonization in the Cooperative Republic of Guyana (formerly British Guiana), Colombia, and Brazil [8–10]. Additional colonies were reported in Brazil in 1970 and 1988 [11,12], but long-term maintenance proved difficult. After nearly 40 years, new free-mating An. darlingi colonies were successfully established in Peru [13,14], Brazil [15], and in French Guiana [16].
The reproductive characteristics of some Anopheles species, such as eurigamy (the ability to mate freely in natural environments), initially limited their colonization in laboratories. However, this challenge was overcome by the development of the force mating technique, first described by [17] and later adapted by [18] and [19]. Larger cages or rooms were also used to facilitate mating swarms [11,12]. After a few generations, the natural selection of stenogamous strains allowed colonies of some Anopheles species to be maintained in smaller cages without the need for forced copulation. However, attempts to establish An. darlingi colonies using force mating techniques were largely unsuccessful.
Earlier attempts to colonize An. darlingi utilized larger cages or rooms and artificial lights to induce mating swarms [11,12]. Initial efforts resulted in colonies lasting only six generations [8], while later studies achieved maintenance for up to ten years [8,11], demonstrating the potential for long-term colonization despite challenges such as low sexual activity and oviposition rates [12]. Since 2010, An. darlingi colonies have been successfully established using induced copulation by light flashes, first described for An. pseudopunctipennis [20], with modifications such as reduced room temperature during inductions. These colonies, maintained for more than six generations, provide crucial insights into the species’ biology and support vector control strategies in the Amazon region. They also enable the testing of insecticides and population suppression or replacement technologies, such as the sterile insect technique (SIT), transgenic mosquito production, and release of insects carrying a dominant lethal gene (RIDL). Additionally, paratransgenesis techniques, which utilize symbiotic bacteria like Asaia, are being explored as potential malaria control strategies.
A recent study demonstrated that under laboratory conditions, the bacterium Delftia tsuruhatensis, found in the gut of Anopheles mosquitoes, can block the development of P. falciparum in An. gambiae s.s. through the production of the metabolite harmane [21]. Studies on the microbiota of An. darlingi, including its midgut and salivary glands, are currently underway to identify symbiotic bacteria suitable for paratransgenesis [22].
In Brazil, the establishment of An. darlingi [15] and An. deaneorum [5] colonies, coupled with successful P. vivax infections, led to the creation of the first Malaria Vectors Production and Infection Platform (Plataforma de Produção e Infecção de Vetores da Malária, PIVEM) in 2020. PIVEM supports research on Anopheles–Plasmodium interactions, including P. vivax liver studies [23,24] and transmission-blocking interventions [25–29].
Here, we provide a comprehensive description of the procedures required to initiate and maintain the large-scale mass rearing of Neotropical Anopheles mosquitoes using flashlight-induction copulation. This step-by-step guide outlines the processes for colony establishment, scaling up production, and maintaining laboratory populations, including successful artificial infection with P. vivax.
Materials and reagents
A. Producing the F1 generation from wild Anopheles darlingi and Anopheles deaneorum
1. Foam box 50 L
2. Foam box 10 L
3. Foam box 5 L
4. Damp towels
5. Black plastic garbage bag 50 L
6. Spherical plastic cups with lid (750 mL) covered by mesh netting and secured with rubber bands and a plastic lid (here called field cages)
7. Cotton pad
8. Sucrose, common table sugar (Açúcar Cristal Barralcool) with a minimum concentration of 99.7%
9. Chicken (live adult Gallus gallus domesticus, 2–3 kg weight)
10. Ice
11. Dichotomous keys proposed by Consoli & Lourenço-de-Oliveira [30]
12. Qualitative filter paper 80 g, 90 mm (Unifil, catalog number: 501.009)
13. Qualitative filter Paper 80 g, 50 cm × 50 cm (Unifil, catalog number: 501.1250)
14. Feather-tip forceps (Bioquip, catalog number: 4748)
15. Dissection forceps (Fine Science Tools Inc., Dumont, #5-Fine)
16. Petri dish (GosselinTM, catalog number: BP93B-101, diameter: 90 mm, height: 14.2 mm)
17. Distilled water
18. 0.22 μm vacuum filtration system (Kasvi, catalog number: K15-1000)
19. 10% sodium hypochlorite (Cinord, 69, https://www.primecirurgica.com.br/hipoclorito-de-sodio-10-kit-com-4-galaos-de-5-litros-cinord-p2816/p)
20. White plastic pans (30.3 × 22.1 × 7.5 cm) (here called rearing pans)
21. Squirt bottle 500 mL
22. Spherical plastic containers (500 mL) (here called pupae containers)
23. Black plastic container (800 mL, 15 × 20 × 4 cm) (here called oviposition containers)
24. Laboratory test sieve (63, 125, and 250 μm) (Bertel, catalog numbers: 350500, 405983, and 500781)
25. Brush size 0
26. Beaker 5 mL, graduated glass
27. Tetra Marine Granules (Tetra®)
28. 3 mL plastic Pasteur pipette
29. Yeast extract power, 100 g (Chemical, catalog number: 8013-01-2 or similar)
30. Tube for mixing yeast, e.g., 50 mL disposable
31. Honey solution (local production, Apicultura Colonial, Vilhena, Rondonia)
32. 1 L clear plastic pitchers with volume markings
33. Sieves of different meshes (commercial)
34. Cages (30 × 30 cm) in aluminum (Horst Armadilhas Ltda-Me, http://www.horstarmadilhas.com.br/)
B. Induced mating by light stimulation to natural copulation
1. Cages (61 × 61 cm, 46 × 46 cm and 30 × 30 cm) in aluminum (Horst Armadilhas Ltda-Me, http://www.horstarmadilhas.com.br/)
2. Black plastic container (800 mL, 15 × 20 × 4 cm) (here called oviposition containers)
3. Chicken (live adult Gallus gallus domesticus, 2–3 kg weight)
4. Cotton pad
5. Honey solution (Local production, Apicultura Colonial, Vilhenal Rondonia)
6. Qualitative filter paper 80 g, 50 cm × 50 cm
C. Anopheles darlingi and Anopheles deaneorum rearing and maintenance for scientific experiments
1. Swine sodium heparin 5,000 U.I./mL (HEPAMAX-S, catalog number: 22021550)
2. Bovine blood
3. Glass bottle, 500 mL
4. Qualitative filter paper 80 g, 50 cm × 50 cm (Unifil, catalog number: 501.1250)
5. Distilled water
6. Squirt bottle 500 mL
7. White plastic pans (30.3 × 22.1 × 7.5 cm) (rearing pans)
8. White plastic containers spherical (500 mL) (pupae containers)
9. Black plastic container (800 mL, 15 × 20 × 4 cm) (oviposition containers)
10. Laboratory test sieve (63, 125, and 250 μm) (Bertel, catalog numbers: 350500, 405983, and 500781)
11. Brush size 0
12. Lab spatula spoon size ø 5mm
13. Beaker 5 mL, graduated glass
14. Tetra Marine Granules (Tetra®)
15. 3 mL plastic Pasteur pipette
16. Yeast extract power, 100 g (Chemical, catalog number: 8013-01-2 or similar)
17. Tube for mixing yeast, e.g., 50 mL disposable
18. Honey solution (local production, Apicultura Colonial, Vilhena, Rondonia)
19. 1 L clear plastic pitchers with volume markings
20. Sieves of different meshes (commercial)
21. Cages (30 × 30 cm) in aluminum (Horst Armadilhas Ltda-Me, http://www.horstarmadilhas.com.br/)
22. Styrofoam cups (180 mL size)
23. Polytetrafluoroethylene (PTFE) seal tape (~80 × 20 × 0.2 mm)
D. Artificial infection by direct membrane feeding assay (DMFA)
1. 6 mL vacuum sodium heparin tubes (BD Vacutainer, catalog number: 367886)
2. 1,000 μL to 200 μL pipette and tips
3. Human AB serum inactivate (human donation)
4. Parafilm “M” roll, 10.16 cm × 38.10 (American, catalog number: PM996)
5. Spherical plastic cups (650 mL) covered by mesh netting and secured with rubber bands and a plastic lid (blood feeding cups)
6. Spherical plastic cups (2,000 mL) covered by mesh netting and secured with rubber bands and a plastic lid (infected cups)
7. 70% ethanol in water
8. Honey solution (local production, Apicultura Colonial, Vilhena, Rondonia)
9. Cotton pad
E. Midgut and salivary gland dissection
1. Glass slides 26 × 76 mm, 1–1.2 mm thickness (PerfectaTM, catalog number: 7105)
2. Coverslips 13 mm, 0.13–0.16 mm thickness (OlenTM, catalog number: K5-0013)
3. Dissection needle 0.20 mm (Rodoz Surgical Instrument CO., catalog number: RS-6083-20)
4. Needle holder for micro dissecting needles (Rodoz Surgical Instrument CO., catalog number: RS-6061)
5. Dissection forceps (Fine Science Tools Inc., Dumont, #5-Fine)
6. Mercury dibromofluorescein disodium salt (Mercurochrome, Sigma-Aldrich, catalog number: M7011-10G)
7. RNA-free disposable pellet pestles (FisherbradTM, catalog number: 12-544-2)
8. DNA LoBind tube 1.5 mL (Eppendorf TM, catalog number: J190474)
9. PBS solution
10. Glass or plastic pestle for 1.5 mL tubes (MerckTM, catalog number: BAF199230001)
11. Neubauer chamber (Olen, catalog number: K5-0111)
12. RPMI-1640 medium (Sigma-Aldrich, catalog number: R5886-100ML)
F. Cleanliness and general maintenance
1. 10% Sodium hypochlorite (Cinord, 69, https://www.primecirurgica.com.br/hipoclorito-de-sodio-10-kit-com-4-galaos-de-5-litros-cinord-p2816/p)
2. 100% lab detergent (Extran MA02 Neutro Merck 5 L or similar)
3. 15% peracetic acid (Spartan®, https://www.spartanbrasil.com.br/produtos/detalhes/83/peraceticfood.html#info)
4. Ethanol (absolute)
Solutions
1. 10% sucrose solution (see Recipes)
2. 0.25% sodium hypochlorite (bleach) solution (see Recipes)
3. 0.5% sodium hypochlorite (bleach) solution (see Recipes)
4. 15% honey solution (see Recipes)
5. 1% yeast solution (see Recipes)
6. 70% ethanol (see Recipes)
7. 2% mercurochrome stock solution (see Recipes)
8. 0.2% mercurochrome solution (see Recipes)
9. 6% lab detergent (see Recipes)
10. 0.02% peracetic acid solution (see Recipes)
Recipes
1. 10% sucrose solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Sucrose | 10% | 5 g |
| H2O | n/a | Fill to 50 mL |
| Total | n/a | 50 mL |
Keep up to 7 days stored at 4 °C. Leave for 1 h at room temperature before use.
2. 0.25% sodium hypochlorite (bleach) solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium hypochlorite solution (10%) | 0.25% | 75 mL |
| ddH2O | n/a | Fill to 3,000 mL |
| Total | n/a | 3,000 mL |
Keep up to 7 days; store at room temperature.
3. 0.5% sodium hypochlorite (bleach) solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium hypochlorite solution (10%) | 0.5% | 50 mL |
| ddH2O | n/a | Fill to 1,000 mL |
| Total | n/a | 1,000 mL |
Keep up to 7 days; store at room temperature.
4. 15% honey solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Honey | 15% | 150 mL |
| ddH2O | n/a | Fill to 1,000 mL |
| Total | n/a | 1,000 mL |
Keep up to 7 days stored at 4 °C. Leave for 1 h at room temperature before use.
5. 1% yeast solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Yeast | 1% | 10 g |
| ddH2O | n/a | Fill to 1,000 mL |
| Total | n/a | 1,000 mL |
Keep up to 7 days stored at 4 °C. Leave for 1 h at room temperature before use.
6. 70% ethanol
| Reagent | Final concentration | Amount |
|---|---|---|
| Ethanol (absolute) | 70% | 700 mL |
| H2O | n/a | Fill to 1,000 mL |
| Total | n/a | 1,000 mL |
Store at room temperature.
7. 2% mercurochrome stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Mercurochrome | 2% | 0.2 g |
| H2O | n/a | Fill to 10 mL |
| Total | n/a | 10 mL |
Store at room temperature.
8. 0.2% mercurochrome solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Mercurochrome 2% | 0.2% | 1 mL |
| H2O | n/a | Fill to 10 mL |
| Total | n/a | 10 mL |
Store at room temperature.
9. 6% lab detergent
| Reagent | Final concentration | Amount |
|---|---|---|
| Lab detergent 100% | 6% | 60 mL |
| H2O | n/a | Fill to 1,000 mL |
| Total | n/a | 1,000 mL |
Store at room temperature.
10. 0.02% peracetic acid solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Peracetic acid solution 15% | 0.02% | 700 μL |
| H2O | n/a | Fill to 500 mL |
| Total | n/a | 500 mL |
Store at room temperature.
Equipment
A. Producing the F1 generation from wild Anopheles darlingi and Anopheles deaneorum
1. BG-malaria traps (BGM) [31]
2. Mouth aspirator (Castro type) (Horst Armadilhas Ltda-Me, http://www.horstarmadilhas.com.br/)
3. InsectaVac vacuum-powered insect aspirator (BioQuip Products, catalog number: 2809B)
4. Stereomicroscope (Leica, model: EZ4)
5. Suction filtration vacuum pump (Nevoni, model: MOD5005)
B. Induced mating by light stimulation to natural copulation
1. Blue stroboscopic light source (Opaluz strobe warming light 30 W)
2. InsectaVac vacuum-powered insect aspirator (BioQuip Products, catalog number: 2809B)
C. Anopheles darlingi and Anopheles deaneorum rearing and maintenance for scientific experiments
1. Hemotek membrane feeding system used with Parafilm membrane (Hemotek®, catalog number: SP6W1-3)
2. Newbauer chamber (Olen, catalog number: K5-0111)
3. Centrifuge 5702 (Eppendorf®, catalog number: 5703000322)
4. InsectaVac vacuum-powered insect aspirator (BioQuip Products, catalog number: 2809B)
5. Stereomicroscope (Leica, catalog number: EZ4)
6. Microscope (Leica, catalog number: DM750)
Procedure
文章信息
稿件历史记录
提交日期: Jan 2, 2025
接收日期: Apr 7, 2025
在线发布日期: Apr 20, 2025
出版日期: May 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Araujo, M. S., Andrade, A. O., Bastos, A. S., Santos, N. A. C., Pontual, J. D. C., Araújo, J. E., Rocha, M. L., Miguel, M. E. R., Costa, A. E. M., Vinetz, J. M., Gazzinelli, R. T. and Medeiros, J. F. (2025). From Colonization to High Production and Plasmodium vivax Infection of Anopheles darlingi and Anopheles deaneorum: a Platform for Malaria Research. Bio-protocol 15(9): e5302. DOI: 10.21769/BioProtoc.5302.
分类
环境生物学 > 寄生生物
医学
微生物学 > 微生物-宿主相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










