- EN - English
- CN - 中文
Applying LFQRatio Normalization in Quantitative Proteomic Analysis of Microbial Co-culture Systems
在微生物共培养体系中应用LFQRatio标准化进行定量蛋白质组学分析
发布: 2025年05月05日第15卷第9期 DOI: 10.21769/BioProtoc.5294 浏览次数: 2307
评审: Hemant Kumar PrajapatiAakanksha J. Sane
Abstract
Quantitative proteomic analysis plays a crucial role in understanding microbial co-culture systems. Traditional techniques, such as label-free quantification (LFQ) and label-based proteomics, provide valuable insights into the interactions and metabolic exchanges of microbial species. However, the complexity of microbial co-culture systems often leads to challenges in data normalization, especially when dealing with comparative LFQ data where ratios of different organisms can vary across experiments. This protocol describes the application of LFQRatio normalization, a novel normalization method designed to improve the reliability and accuracy of quantitative proteomics data obtained from microbial co-cultures. The method was developed following the analysis of factors that affect both the identification of proteins and the quantitative accuracy of co-culture proteomics. These include peptide physicochemical characteristics such as isoelectric point (pI), molecular weight (MW), hydrophobicity, dynamic range, and proteome size, as well as shared peptides between species. We then created a normalization method based on LFQ intensity values named LFQRatio normalization. This approach was demonstrated by analysis of a synthetic co-culture of two bacteria, Synechococcus elongatus cscB/SPS and Azotobacter vinelandii ΔnifL. Results showed enhanced accuracy of differentially expressed proteins, allowing for more reliable biological interpretation. This protocol provides a reliable and effective tool with wider application to analyze other co-culture systems to study microbial interactions.
Key features
• Assessment of factors affecting the quantitative accuracy of co-culture proteomics.
• Provides a LFQRatio normalization method for label-free quantification of microbial co-cultures.
• Recommendations for co-culture proteomics for mixed microbial populations.
Keywords: Proteomics (蛋白质组学)Background
Microbial co-cultures are valuable models for studying interactions between different microbial species, revealing insights into symbiotic relationships, competition, and metabolic cooperation [1–3]. Quantitative proteomics provides a critical tool that allows for the comprehensive analysis of protein dynamics in various biological systems, including microbial co-cultures.
Quantitative analysis in proteomics has largely shifted to label-free methods due to the rapidly increasing sensitivity of liquid chromatography (LC) and mass spectrometry hardware and the accuracy of proteomics data analysis tools [4]. Label-free quantification (LFQ) has advantages such as being cost-saving, requiring less stringent chemicals for extraction buffers, and presenting no limitations on the number of samples compared to label-based methods [5]. However, sample complexity and variations in protein abundance can make data interpretation difficult.
Many attempts have been made to deal with systematic biases among samples and to improve the accuracy of LFQ proteomics, including the creation of new algorithms [6–8]. However, there are challenges in applying these common workflows to synthetic co-cultures, i.e., when several strain types are cultivated together. This is particularly important when comparing conditions with highly variable cell-type ratios, as this will cause large differences in protein abundance between samples [9]. Therefore, a method for interpreting the contribution of two distinct protein groups in a sample in quantitative proteomics analyses remains to be established.
To address these challenges, we started by analyzing the physicochemical properties and factors related to protein quantification between two co-cultured species. Then, we developed a new normalization method named LFQRatio, designed for the quantitative proteomic analysis of microbial co-culture systems (Figure 1). The LFQRatio normalization method is based on a more accurate LFQ intensity-based quantification approach [10, 11], which addresses the effects of changes in cell proportions between co-cultured microorganisms and facilitates comparisons of protein abundance under different conditions. This method enables more accurate identification of differentially expressed proteins, which is crucial for deciphering dynamics within microbial co-cultures (Figure 2).
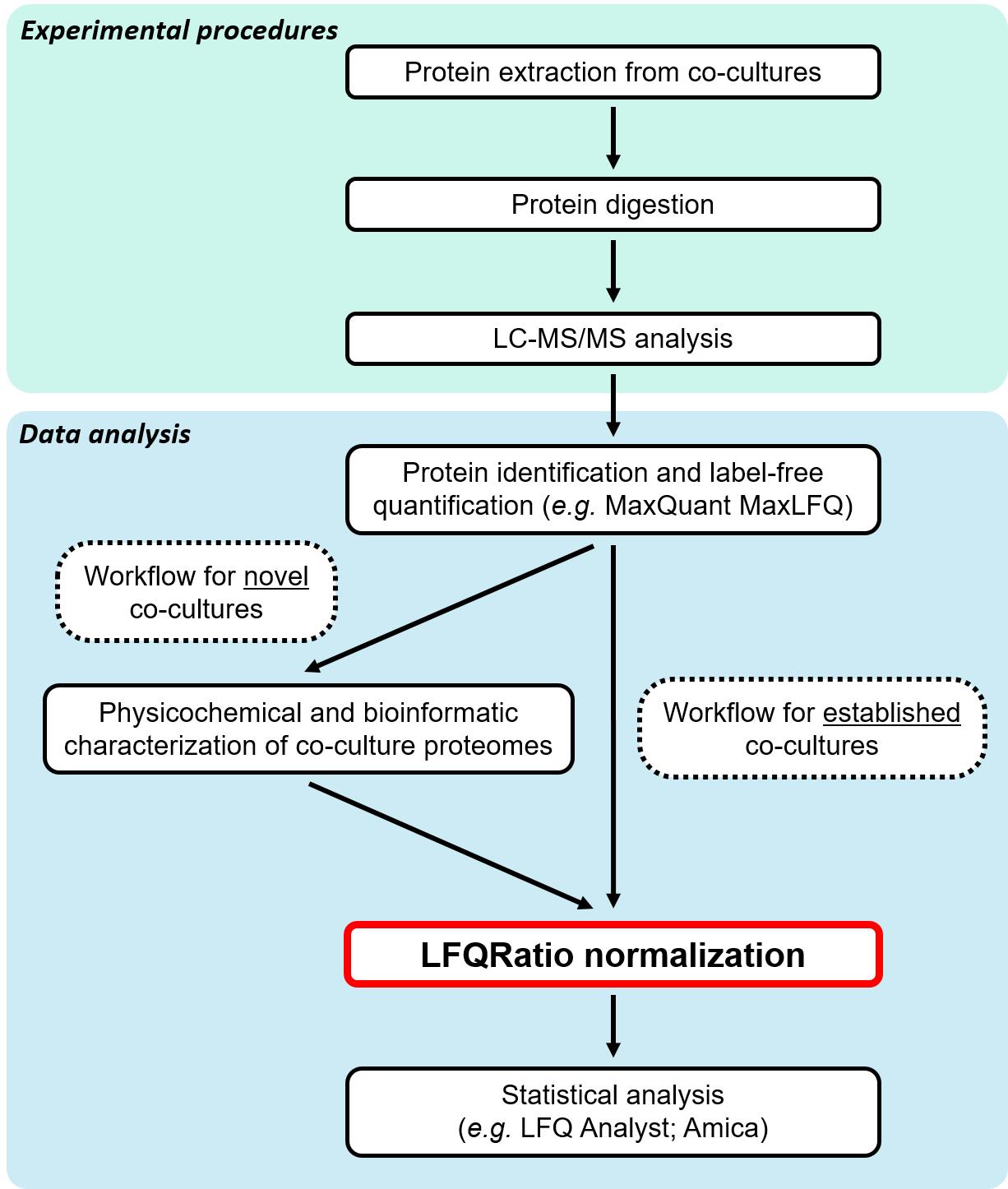
Figure 1. Label-free quantitative proteomics workflow with LFQRatio normalization for the analysis of co-cultures
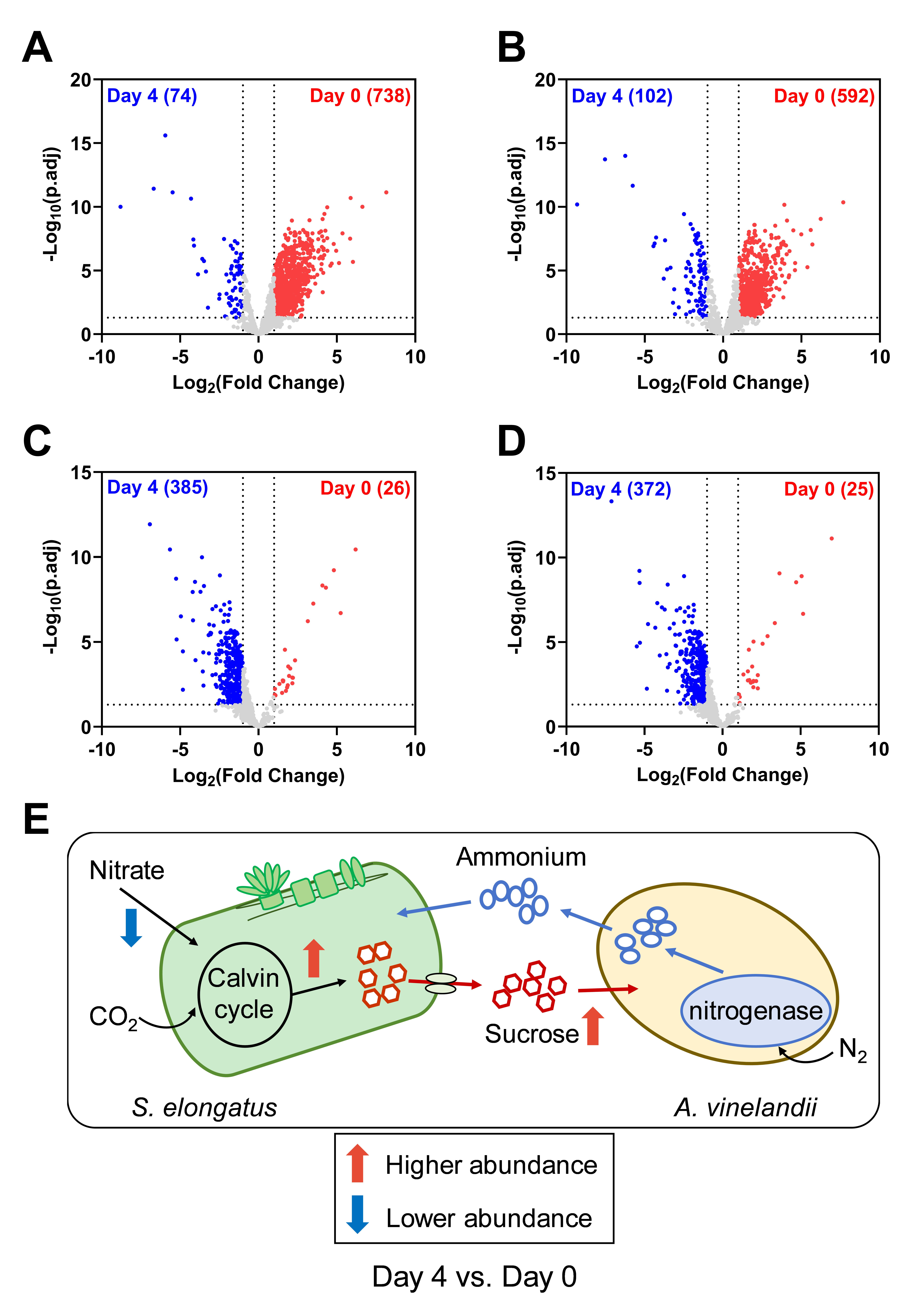
Figure 2. Example results obtained when including LFQRatio normalization in our label-free quantitative proteomics analysis pipeline for an S. elongatus–A. vinelandii co-culture. (A–D) Volcano plots showing differentially enriched proteins (DEPs) between Day 0 and Day 4 of an S. elongatus–A. vinelandii co-culture, with and without LFQRatio normalization. (A) DEPs of S. elongatus between Day 0 and Day 4 without LFQRatio normalization. (B) DEPs of S. elongatus between Day 0 and Day 4 with LFQRatio normalization. (C) DEPs of A. vinelandii between Day 0 and Day 4 without LFQRatio normalization. (D) DEPs of A. vinelandii between Day 0 and Day 4 with LFQRatio normalization. Each data point represents an individual protein. Proteins significantly enriched on Day 0 and Day 4 are shown in red and blue, respectively. Differential expression analysis was performed using LFQ Analyst using the following parameters: p-value cutoff = 0.05; Log2(fold change) cutoff = 1; imputation = Perseus style. (E) Schematic showing the changes in cross-feeding dynamics between Day 4 and Day 0 in an S. elongatus–A. vinelandii co-culture uncovered using LFQRatio normalization. On Day 4, nitrate uptake transporters in S. elongatus were less abundant, while proteins involved in sucrose production in S. elongatus and sucrose import in A. vinelandii were enriched, compared to Day 0. These biological conclusions were able to be drawn by applying LFQRatio normalization to the LFQ intensity values acquired using MaxQuant MaxLFQ; for more details, see Shi et al. [11].
Materials and reagents
Biological materials
1. Bacterial monoculture 1: Synechococcus elongatus PCC 7942 cscB/SPS monoculture (from Abramson et al. [12]; kindly provided by Prof. Daniel Ducat). For growth monitoring information, see General notes
2. Bacterial monoculture 2: Azotobacter vinelandii DJ ΔnifL monoculture (from Ortiz-Marquez et al. [13]; kindly provided by Prof. Leonardo Curatti)
3. Bacterial co-culture (combination of bacterial monocultures 1 and 2): Synechococcus elongatus cscB/SPS and Azotobacter vinelandii ΔnifL co-culture
Caution: These strains are genetically modified and so need to be handled and disposed of according to the recommended safety guidelines of your institute.
Reagents
1. Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: 71725)
2. Phosphate-buffered saline (PBS) tablets (Millipore, catalog number: 6500-OP)
3. 100× Halt protease inhibitor cocktail (Thermo Scientific, catalog number: 78446)
4. 425–600 µm glass beads, acid-washed (Sigma-Aldrich, catalog number: G8772)
5. Urea, proteomics grade (Thermo Scientific, catalog number: 29700)
6. Trizma® base (Sigma-Aldrich, catalog number: T1503)
7. Buffers for adjusting pH (e.g., NaOH/HCl) (Sigma-Aldrich, catalog number: 06203/258148)
8. DL-Dithiothreitol (DTT) (Promega, catalog number: V3151)
9. Water, HPLC grade (Fisher, catalog number: W/0106/PB17)
10. Iodoacetamide (IAA) (Sigma-Aldrich, catalog number: 1149)
11. Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C3306)
12. Trifluoroacetic acid, HPLC grade (TFA) (Sigma-Aldrich, catalog number: 15608360 or similar)
13. Sequencing-grade modified trypsin (Promega, catalog number: V5111)
14. Formic acid, LC-MS grade (Thermo Scientific, catalog number: 85178)
15. Acetonitrile (ACN), HPLC grade (Fisher, catalog number: A/0627/17)
16. Methanol (MeOH), HPLC grade (Fisher, catalog number: M/4056/17)
Solutions
1. 1 M Tris-HCl (pH 8.5) (see Recipes)
2. 1 M DTT (see Recipes)
3. Lysis buffer (see Recipes)
4. Urea buffer (see Recipes)
5. 100 mM iodoacetamide (IAA) (see Recipes)
6. 1 mg/mL trypsin stock (see Recipes)
7. 100 mM CaCl2 (see Recipes)
8. 50 mM Tris-HCl (pH 8.5)/10 mM CaCl2 (see Recipes)
9. MS loading buffer (see Recipes)
10. 80% ACN (see Recipes)
11. MS mobile phase (see Recipes)
Recipes
Note: Storage conditions are room temperature unless otherwise stated.
1. 1 M Tris-HCl (pH 8.5)
Weigh 12.114 g of Tris and dissolve in 80 mL of HPLC-grade water. Lower the pH to 8.5 with HCl and top up to 100 mL with HPLC-grade water.
2. 1 M DTT
Dissolve 1.542 g of DTT in 10 mL of HPLC-grade water. Aliquot and store at -20 °C or use immediately.
Note: DTT oxidizes in air, so the solution must be prepared fresh or frozen aliquots must be used immediately and then discarded.
3. Lysis buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| SDS | 2% (w/v) | 400 mg |
| 1 M Tris Tris-HCl (pH 8.5) | 40 mM | 0.8 mL |
| 1 M DTT | 60 mM | 1.2 mL |
| HPLC-grade water | Up to 20 mL |
4. Urea buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Urea | 8 M | 240 mg |
| 1 M Tris-HCl (pH 8.5) | 100 mM | 50 µL |
| 1 M DTT | 5 mM | 2.5 µL |
| HPLC-grade water | Up to 500 µL |
Note: We recommend using 1.5 mL LoBind Eppendorf tubes to prepare the solution. Always make up fresh urea solution. When in solution, urea degrades within a few hours into a compound that can alter the composition of your protein sample.
5. 100 mM iodoacetamide (IAA)
Dissolve 0.0185 g of iodoacetamide in 1 mL of HPLC-grade water.
Note: Always prepare fresh as IAA is light-sensitive. Wrap in foil once prepared.
6. 1 mg/mL trypsin stock
Add 20 µL of resuspension buffer (provided by the trypsin manufacturer) to one vial of lyophilized sequencing-grade modified trypsin (20 µg) to generate the trypsin stock. Store at -80 °C for up to 1 month.
7. 100 mM CaCl2
Dissolve 1.47 g of CaCl2 in 100 mL of HPLC-grade water.
8. 50 mM Tris-HCl (pH 8.5)/10 mM CaCl2
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl (pH 8.5) | 50 mM | 1 mL |
| 100 mM CaCl2 | 10 mM | 2 mL |
| HPLC-grade water | 17 mL |
9. MS loading buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| ACN | 3% (v/v) | 30 µL |
| TFA | 0.1% (v/v) | 1 µL |
| HPLC-grade water | 969 µL |
Caution: Prepare in a fume cabinet.
10. 80% ACN
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| ACN | 80% (v/v) | 800 mL |
| HPLC-grade water | 200 mL |
Caution: Prepare in a fume cabinet.
11. MS mobile phase
Solvent A
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Formic acid | 0.1% (v/v) | 1 mL |
| HPLC-grade water | 999 mL |
Solvent B
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Formic acid | 0.1% (v/v) | 1 mL |
| 80% ACN | 999 mL |
Caution: Prepare in a fume cabinet.
Laboratory supplies
1. 1,000 µL pipette tips
2. 200 µL pipette tips
3. 10 µL pipette tips
4. 50 mL sterile conical tubes (Falcon, catalog number: 352070)
5. 1.5 mL protein LoBind tubes (Eppendorf, catalog number: 0030108116)
6. 2D Clean-Up kit (Cytiva, formerly GE Healthcare Life Sciences, catalog number: 80-6484-51)
7. PierceTM C18 spin columns (Thermo Scientific, catalog number: 89870)
8. 0.3 µL PP snap ring HPLC vials (VWR, catalog number: 548-0120A)
9. ND11 open-top PE HPLC vial snap caps (VWR, catalog number: 548-3203A)
Equipment
1. Pipettes (p1000, p200, p10, p2.5) (Eppendorf)
2. Spectrophotometer (Jenway, model: 6305)
3. Temperature-controlled centrifuge (e.g., Thermo Scientific, model: SL 1ER)
4. Vortex with attachment for multiple microfuge tubes (Scientific Industries, model: Vortex-Genie 2)
5. Heated water bath (e.g., Grant, model: JB Nova)
6. Ultrasonic water bath (Fisherbrand, model: FB15051)
7. Nanodrop spectrophotometer (Thermo Scientific, model: Nanodrop 2000)
8. Incubator capable of maintaining 30–37 °C (e.g., Thermo Scientific, model: MaxQ 6000)
9. Vacuum concentrator (Eppendorf, model: Concentrator Plus)
10. Nano-flow liquid chromatography (Thermo Fisher Scientific, model: U3000 RSLCnano)
11. Hybrid quadrupole-orbitrap mass spectrometer (Thermo Fisher Scientific, model: Q Exactive HF)
12. Easy-Spray C18 column, 75 µm × 50 cm, Pepmap RSLC, 2 µm, 100 Å (Thermo Scientific, catalog number: E5903)
Software and datasets
1. Uniprot (https://www.uniprot.org/, 02/03/2020)
2. MaxQuant (2.0.3.0)
3. RStudio (4.2.0)
4. LFQ-Analyst (https://analyst-suite.monash-proteomics.cloud.edu.au/apps/lfq-analyst/, 08/09/2023)
5. All code has been deposited to GitHub: https://github.com/Mengxun-Shi/bio-protocol (01/13/2025)
Procedure
文章信息
稿件历史记录
提交日期: Jan 13, 2025
接收日期: Mar 23, 2025
在线发布日期: Apr 15, 2025
出版日期: May 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Shi, M., Evans, C. A., McQuillan, J. L., Noirel, J. and Pandhal, J. (2025). Applying LFQRatio Normalization in Quantitative Proteomic Analysis of Microbial Co-culture Systems. Bio-protocol 15(9): e5294. DOI: 10.21769/BioProtoc.5294.
分类
生物信息学与计算生物学
微生物学 > 微生物蛋白质组学 > 全生物体
系统生物学 > 微生物组学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













