- EN - English
- CN - 中文
Isolation and Culture of Primary Pericytes from Mouse
小鼠原代周细胞的分离和培养
发布: 2025年04月20日第15卷第8期 DOI: 10.21769/BioProtoc.5288 浏览次数: 2948
评审: Pilar Villacampa AlcubierreJosé M. DiasHaixia Xu
Abstract
Pericytes are essential for tissue homeostasis, functioning to regulate capillary blood flow. Dysfunctional pericytes are implicated in various pathologies, including cancer progression. Despite their important function in both health and disease, pericytes remain understudied due to a lack of robust model systems that accurately reflect their in vivo biology. Here, we present a comprehensive protocol for isolating and culturing primary pericytes from murine lung, brain, bone, and liver tissues, based on NG2 expression using an antibody-conjugated magnetic bead approach. Our protocol emphasizes the importance of physiological oxygen tension during ex vivo culture (10% O2 for lung pericytes and 5% O2 for brain, bone, and liver pericytes). These conditions stabilize the expression of characteristic pericyte markers at both the transcriptional and protein levels. Importantly, we optimized growth conditions to limit the expression of the plasticity factor Klf4 in order to prevent spontaneous phenotypic switching in vitro. This protocol provides a reliable and reproducible method for obtaining pericytes suitable for high-throughput analyses in order to explore pericyte biology in both physiological and pathological contexts.
Key features
• Isolation of primary pericytes from mouse lung, brain, bone, and liver.
• Emphasis on physioxic culturing conditions to better maintain pericyte phenotype.
• Representative of pericyte biology in both health and disease contexts.
Keywords: Pericytes (周细胞)Graphical overview
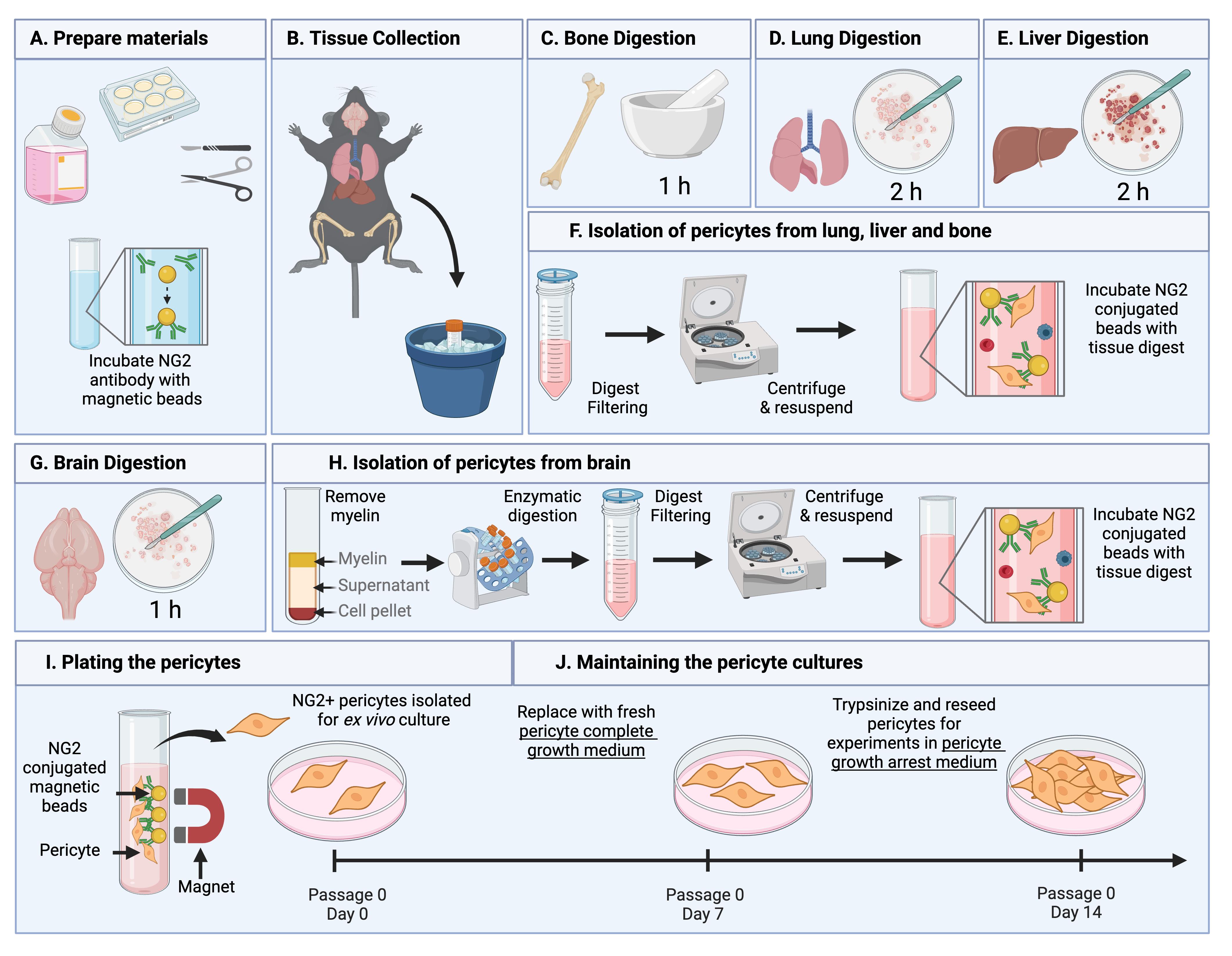
Summary of the pericyte isolation protocol. (A) Before beginning, dissection tools are sterilized, pericyte medium is equilibrated, cell culture plates are coated with collagen, and NG2 antibody–conjugated Dynabeads are prepared. (B) Organs of interest are collected from 12-week-old C57BL/6 mice following euthanasia by isoflurane and cervical dislocation. (D–E) Bone, lung, and liver tissues undergo organ-specific digestion steps prior to isolation described in (F). (F) Digest from the lung, bone, and liver are filtered, centrifuged, and incubated with NG2-conjugated beads. (G) Brain tissues undergo digestion steps. (H) Digest from the brain undergoes myelin removal and further digestion before being filtered, centrifuged, and incubated with NG2 conjugated beads. (I–J) The bead-bound NG2-expressing cells are isolated and washed using a magnet and plated for expansion under optimized pericyte culturing conditions. Figure created in Biorender.com.
Background
Pericytes are integral components of the microvasculature, forming direct interactions with endothelial cells and other mural cells to maintain tissue homeostasis by regulating capillary blood flow and endothelial barrier function [1,2]. Dysfunction or loss of pericytes has been implicated in various pathologies, including aging, ischemic disease, fibrosis, neurodegeneration, and cancer [3–8].
Genetic mouse models have been invaluable tools in elucidating pericyte function during development and disease [9–11]. However, in vitro tools are lacking despite being essential for high throughput and mechanistic studies. This is partly due to the heterogeneity of pericyte origins, functions, and identification markers, as well as their intrinsic plasticity, which can result in undesired lineage differentiation [1,2,7,12–16]. Unfortunately, existing in vitro models present significant limitations. For example, immortalized pericyte cell lines exhibit constitutive proliferation, a hallmark of perivascular activation not typically observed in quiescent pericytes in vivo [7,17,18]. Alternatively, pericytes derived from induced pluripotent stem cells (iPSCs) require complex differentiation protocols and highly specific culturing conditions, making these protocols less accessible [19,20].
Primary pericyte cultures present a more physiologically relevant alternative tool, as they have the potential to retain tissue-specific characteristics and better reflect in vivo biology. While isolation of perivascular cells has been achieved, these methods lack standardization, which hinders reproducibility [21]. A common cell isolation approach involves enzymatic digestion of tissues followed by purification via fluorescence-activated cell sorting (FACS) or immunomagnetic separation. However, despite successful isolation, cell yield remains a challenge. For example, Lee et al. described a protocol for isolating murine cardiac pericytes, but its high cell input requirement and resulting low yield (1.1%) present significant drawbacks [22]. Low pericyte yield during isolation could be overcome with ex vivo expansion; however, their high differentiation capacity poses an additional challenge. In the development of this protocol, we sought to uncover the culture conditions that would support pericyte expansion while retaining their identity and function, a problem that has been overcome in the culture of other plastic cell populations, i.e., iPSCs, by tightly regulating oxygen tension [23–26].
To address the current limitations in pericyte research, we developed a standardized protocol for isolating and culturing primary murine pericytes from the lung, brain, bone, and liver under physiologically relevant oxygen conditions [27–29]. This approach preserved pericyte identity and function in both homeostatic conditions and recapitulated important pericyte phenotypic switching that has been observed in vivo in response to tumors [7]. This protocol uses NG2 as a marker to selectively isolate pericytes, as it is continuously expressed on arteriolar and mid-capillary pericytes, allowing the capture of multiple pericyte subtypes [30]. However, some pericyte populations, such as those associated with post-capillary venules, may not express NG2 [30] and could be underrepresented, which is a limitation of this method. Additionally, NG2 is expressed by other cell types including oligodendrocyte precursor cells in the brain [31], necessitating validation of pericyte purity in culture. Here, we provide a robust protocol for studying pericyte biology in health and disease.
Materials and reagents
Biological materials
1. 12-week-old female C57BL/6 mice used for pericyte isolation
Reagents
1. 0.05% trypsin-EDTA (Thermo Fisher Scientific, catalog number: 25300062)
2. Acetic acid (Millipore Sigma, catalog number: A6283)
3. Bovine serum albumin (BSA) (Millipore Sigma, catalog number: 12657)
4. Collagen from rat tail (Millipore Sigma, catalog number: C7661)
5. Collagenase A (Millipore Sigma, catalog number: 11088793001)
6. Dispase II (Millipore Sigma, catalog number: 4942078001)
7. DNase1 (Millipore Sigma, catalog number: D4263)
8. Dulbecco’s modified Eagle’s medium (DMEM), low glucose (Millipore Sigma, catalog number: D6046)
9. Sheep anti-rat IgG Dynabeads (Thermo Fisher Scientific, catalog number: 11035)
10. Endothelial cell growth supplement (R&D, catalog number: 390599)
11. Fetal bovine serum (FBS) [GeminiBio, catalog number: 900-208-500, lot tested (lot no. A120030)]
12. Ham’s F12 nutrient mix (Thermo Fisher Scientific, catalog number: 11765047)
13. Heparin (Millipore Sigma, catalog number: H3149)
14. HEPES (1 M) (Thermo Fisher Scientific, catalog number: 15630080)
15. MEM non-essential amino acids (100×) (Thermo Fisher Scientific, catalog number: 11140050)
16. CO2-O2-N2 (5-5-90) compressed gas mix (Roberts Oxygen)
17. NG2 monoclonal antibody (546930) (Thermo Fisher Scientific, catalog number: MA5-24247)
18. Compressed nitrogen gas (Roberts Oxygen)
19. PBS, pH7.4 (Thermo Fisher Scientific, catalog number: 10010023)
20. Penicillin-Streptomycin (Thermo Fisher Scientific, catalog number: 15070063)
21. Sodium pyruvate (100 mM) (Thermo Fisher Scientific, catalog number: 11360070)
Solutions
1. NG2 antibody resuspension (see Recipes)
2. Collagenase A stock solution (see Recipes)
3. Dispase II stock solution (see Recipes)
4. DNase1 stock solution (see Recipes)
5. Heparin stock solution (see Recipes)
6. 0.1% BSA/PBS (see Recipes)
7. 20% BSA in DMEM (see Recipes)
8. Collagen stock solution (see Recipes)
9. Collagen working solution (see Recipes)
10. Pericyte basal medium (see Recipes)
11. Pericyte complete growth medium (see Recipes)
12. Pericyte growth arrest medium (see Recipes)
Recipes
1. NG2 antibody resuspension
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NG2 antibody | 0.5 mg/mL | 100 μg |
| Sterile PBS | n/a | 200 μL |
| Total | n/a | 200 µL |
Once dissolved, store at -20 °C.
2. Collagenase A stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Collagenase A | 100 mg/mL | 500 mg |
| Sterile PBS | n/a | 5 mL |
| Total | n/a | 5 mL |
Calcium- and magnesium-free PBS is used to prevent enzyme inhibition. Collagenase may take up to 20 min to fully dissolve. Keep the solution on ice and invert the vial at regular intervals to mix. Aliquot and store at -20 °C for up to 6 months. To produce the working concentration, dilute the stock in PBS or low-glucose DMEM to a final concentration of 2 mg/mL.
3. Dispase II stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Dispase II | 10 mg/mL | 1 g |
| HEPES buffered saline (pH 7.4) | 50 mM HEPES, 150 mM NaCl | 100 mL |
| Total | n/a | 100 mL |
Once dissolved, aliquot and store at -20 °C for up to 2 months. To produce the working concentration, dilute the stock in low-glucose DMEM to a final concentration of 1 mg/mL.
4. DNase1 stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DNase1 | 1 mg/mL | 1 mg |
| Sterile deionized water | n/a | 1 mL |
| Total | n/a | 1 mL |
Once dissolved, aliquot and store at -20 °C for 1 month. To produce the working concentration, dilute the stock in low-glucose DMEM to a final concentration of 10 μg/mL.
5. Heparin stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Heparin | 10 mg/mL | 1 g |
| Sterile deionized water | n/a | 100 mL |
| Total | n/a | 100 mL |
Aliquot and store at -20 °C for up to 2 years. To produce the working concentration, dilute the stock in PBS to a final concentration of 10 µg/mL.
6. 0.1% BSA/PBS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 15 mM | 0.1 g |
| PBS | n/a | 100 mL |
| Total | n/a | 100 mL |
Once dissolved, filter the solution through a 0.2 μm filter and store at 4 °C for up to 1 month.
7. 20% BSA in DMEM
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 3 M | 20 g |
| DMEM low glucose | n/a | 100 mL |
| Total | n/a | 100 mL |
For brain digestion, a solution of 20% BSA in low-glucose DMEM is required [29]. BSA will dissolve on a roller at 37 °C. Once dissolved, filter the solution through a 0.2 μm filter. 10 mL is needed per batch of 3–6 organs.
8. Collagen stock solution (for cell culture plate coating)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Rat collagen type I | 3 mg/mL | 25 mg |
| 0.1 M acetic acid | 0.1 M | 8,333 μL |
| Total | n/a | 8,333 μL |
Collagen stock should be prepared in 0.1 M acetic acid at a concentration of 3 mg/mL. The collagen in acetic acid should be placed on a roller at room temperature for 2–3 h, followed by overnight rolling at 4 °C. Once dissolved, store the collagen stock at 4 °C.
9. Collagen working solution (for cell culture plate coating)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Collagen stock (Recipe 8) | 0.06 mg/mL | 1 mL |
| Sterile deionized water | n/a | 49 mL |
| Total | n/a | 50 mL |
Before coating the plates, 3 mg/mL collagen stock needs to be diluted 1:50 in sterile deionized water to a final concentration of 0.06 mg/mL. Coat tissue culture plates with collagen working solution (enough to cover the entire surface, i.e., 500 μL per well of a 6-well plate) at 37 °C for at least 2 h before seeding pericytes. Plates can be prepared with collagen at a maximum of one day before pericyte isolation and kept at 37 °C.
10. Pericyte basal medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM | n/a | 375 mL |
| Ham’s F12 nutrient mix | n/a | 375 mL |
| Sodium pyruvate (100 mM) | 2 mM | 20 mL |
| HEPES (1 M) | 20 mM | 20 mL |
| MEM non-essential amino acids (100×) | 1× | 10 mL |
| Total | n/a | 800 mL |
Filter sterilize using vacuum filter units and store at 4 °C for up to 1 month. This pericyte basal medium recipe is the same as that reported for endothelial cell isolation and expansion by Reiterer et al. [29] and Sandovici et al. [32].
11. Pericyte complete growth medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Pericyte basal medium (Recipe 10) | n/a | 38 mL |
| FBS | 20% | 10 mL |
| Endothelial cell growth supplement (50×) | 1× | 1 mL |
| Penicillin/Streptomycin | 1% | 0.5 mL |
| Total | n/a | 50 mL |
Prepare the required volume fresh. Ensure the pericyte basal medium is equilibrated to the appropriate oxygen tension 24 h in advance. Endothelial cell growth supplement should be aliquoted and stored at -20 °C; once aliquots are thawed do not refreeze and use within 1 week. The pericyte complete growth medium composition is the same as that reported for endothelial cell isolation and expansion by Reiterer et al. [29] and Sandovici et al. [32] with the exception of heparin, which is not included.
12. Pericyte growth arrest medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Pericyte basal medium (Recipe 10) | n/a | 48.5 mL |
| FBS | 2% | 1 mL |
| Penicillin/Streptomycin | 1% | 0.5 mL |
| Total | n/a | 50 mL |
Prepare the required volume fresh. Ensure the basal medium is equilibrated to the appropriate oxygen tension 24 h in advance.
Laboratory supplies
1. 15 mL sterile conical tubes (Alkali Scientific Inc, catalog number: CN5600-NEST)
2. 50 mL sterile conical tubes (Alkali Scientific Inc, catalog number: CN5602-NEST)
3. 1.7 mL microcentrifuge tubes (Corning, catalog number: 3620)
4. Tissue culture treated 6-well plates (Alkali Scientific Inc, catalog number: TP9006)
5. 70 μm nylon cell strainer (Fisher Scientific, catalog number: 22-363-548)
6. 96-well cell culture plate (Alkali Scientific Inc, catalog number: TPN1096)
7. 96-well clear bottom black cell culture plate (Fisher Scientific, catalog number: 07-200-567)
8. Cell culture dish 100 × 20 mm (Alkali Scientific Inc, catalog number: TD0100)
9. Sterile scalpels (Fisher Scientific, catalog number: 12-460-454)
10. Filter paper (Millipore Sigma, catalog number: WHA1001240)
11. Vacuum filter units (Fisher Scientific, catalog number: FB12566506)
12. 5 mL serological pipettes (Sarstedt, catalog number: 86.1253.001)
13. 10 mL serological pipettes (Sarstedt, catalog number: 86.1254.001)
14. 50 mL serological pipettes (Sarstedt, catalog number: 86.1256.001)
15. 20 μL filter pipette tips (Alkali Scientific Inc, catalog number: FLT-20)
16. 200 μL filter pipette tips (Alkali Scientific Inc, catalog number: FLT-200
17. 1,000 μL filter pipette tips (Alkali Scientific Inc, catalog number: FLT-1000)
18. 25 cm2 cell culture flask with vented cap (Alkali Scientific Inc, catalog number: TV0025)
Equipment
1. Graefe forceps (Roboz Surgical Store, catalog number: RS-5135)
2. Micro dissecting scissors (Roboz Surgical Store, catalog number: RS-5906SC)
3. Rainin pipette kit (Rainin, catalog number: 30456872)
4. Pipette controller (VWR, catalog number: 89009-344)
5. Mortar (Millipore Sigma, catalog number: Z247464)
6. Pestle (Millipore Sigma, catalog number: Z247502)
7. Refrigerator (4 °C)
8. Sorvall X4 Pro centrifuge (Thermo Fisher Scientific, catalog number: 75009505)
9. Water bath (Thermo Fisher Scientific, catalog number: TSGP2S)
10. Automated cell counter (DeNovix, model: DeNovix CellDrop FL)
11. Freezer (-20 °C)
12. BioTek Lionheart FX automated microscope (Agilent, catalog number: LFXW-SN)
13. Physioxia tissue culture incubator (Thermo Fisher Scientific, catalog number: 51033597)
14. Hypoxia cabinet with O2 controller (Coy Lab Products); hypoxia unit is placed inside a standard tissue culture incubator (Thermo Fisher Scientific, catalog number: 51033544)
15. Magnetic separation rack, 1.5 mL tubes (EpiCypher, catalog number: 10-0012)
16. Nutating Mixer (Corning, catalog number: 6720)
17. Rocking oven/incubator (Boekel Scientific, catalog number: 136400)
18. Zeiss Primovert inverted cell culture microscope with Axiocam 208 (Zeiss, catalog number: 415510-1101-000)
Procedure
文章信息
稿件历史记录
提交日期: Feb 6, 2025
接收日期: Mar 23, 2025
在线发布日期: Apr 7, 2025
出版日期: Apr 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
McErlain, T., Branco, C. M. and Murgai, M. (2025). Isolation and Culture of Primary Pericytes from Mouse. Bio-protocol 15(8): e5288. DOI: 10.21769/BioProtoc.5288.
分类
癌症生物学 > 侵袭和转移 > 细胞生物学试验 > 细胞分离和培养
细胞生物学 > 细胞分离和培养 > 细胞分离 > 免疫磁珠
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link