- EN - English
- CN - 中文
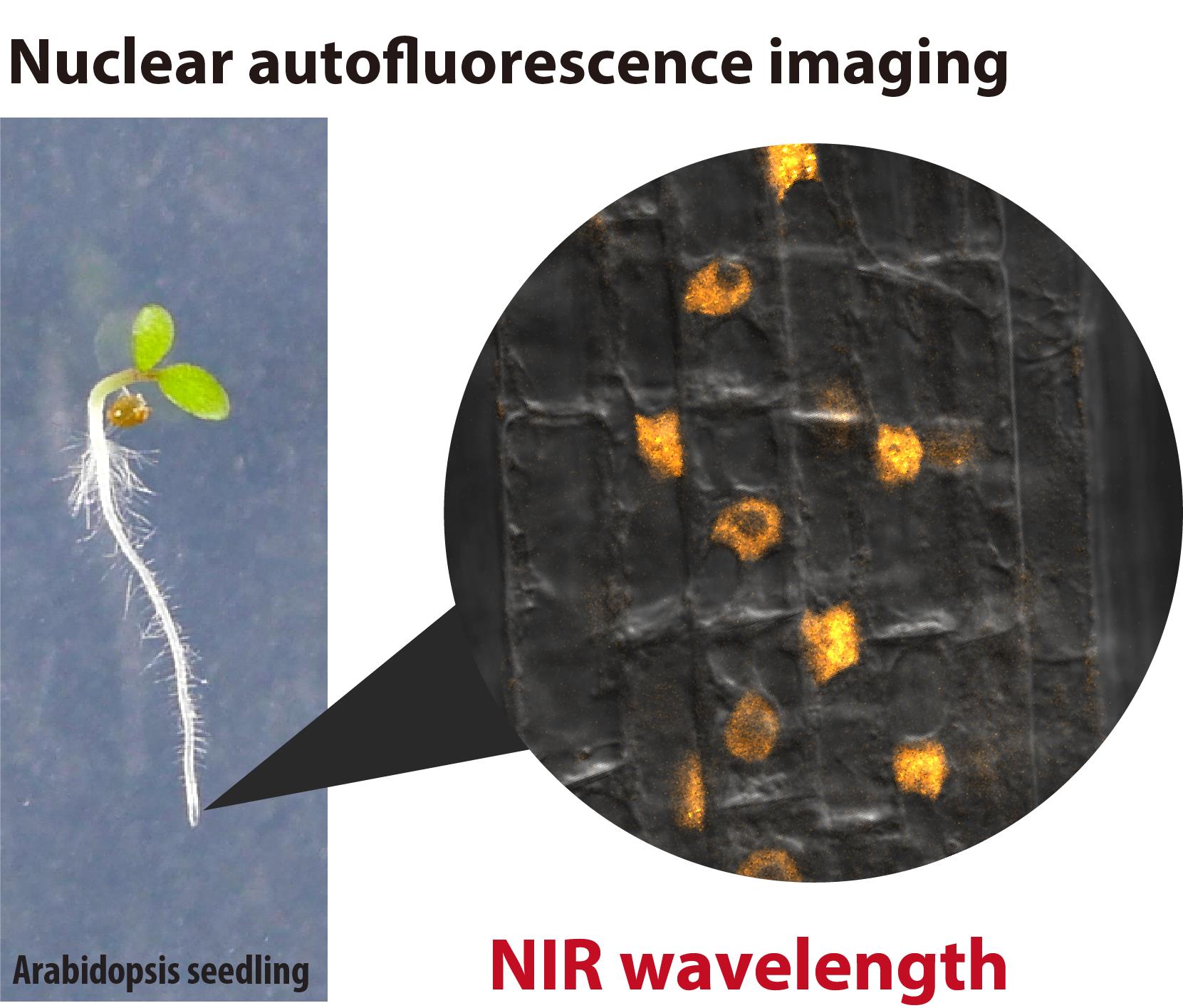
Near-Infrared Autofluorescence Imaging of Nuclei in Living Plant Roots
活体植物根部细胞核的近红外自发荧光成像
发布: 2025年04月20日第15卷第8期 DOI: 10.21769/BioProtoc.5274 浏览次数: 2133
评审: Kentaro TamuraAnonymous reviewer(s)
Abstract
In live-cell imaging, autofluorescence is often regarded as a negative factor that interferes with the accurate visualization of target fluorescence due to a phenomenon known as crosstalk. However, autofluorescence has also been effectively utilized as an organellar marker. For instance, the intense autofluorescence of chlorophyll in the red wavelength is widely used to visualize chloroplasts, the photosynthetic organelle in plants. Recently, we demonstrated that nuclei in plant cells emit phytochrome-derived autofluorescence in the red to infrared wavelength range, which can be visualized by a conventional confocal microscope equipped with a 640 nm laser. Here, we present protocols for growing plants and conducting confocal imaging of the near-infrared autofluorescence of nuclei in Arabidopsis thaliana.
Key features
• Less invasive live-cell imaging of plant nuclei using near-infrared (NIR) autofluorescence derived from phytochrome, a photoreceptor in plants.
• Fluorescence live-cell imaging of non-model plants.
• Imaging of nuclei in root cells and pollen.
Keywords: Autofluorescence imaging (自发荧光成像)Graphical overview
Background
Autofluorescence is extensively utilized as a fluorescence marker for specific compounds or organelles in plant science [1,2]. For instance, the phenolic polymer lignin, located in the cell wall, emits green fluorescence, which is used to visualize the xylem and Casparian strip [3]. Similarly, chlorophyll exhibits prominent autofluorescence in the red wavelength range (600–700 nm, peaking at 675–680 nm) [2]. This chlorophyll autofluorescence is frequently employed to determine the subcellular localization of plastids and chloroplasts [4]. However, the intense autofluorescence of chloroplasts poses a challenge for utilizing fluorescent dyes and proteins that emit in the red to near-infrared (NIR) range for imaging in shoot tissues. Recently, we discovered that plant nuclei emit fluorescence within the 650–720 nm range [5]. This autofluorescence is likely derived from phytochrome, a photoreceptor. The phytochrome-derived autofluorescence can be utilized in confocal imaging to visualize nuclei and intranuclear photobodies in living plant cells. Phytochrome B, a subclass of plant phytochromes, is also known as a temperature sensor that integrates light and temperature signals into plant photomorphogenesis and thermomorphogenesis [6]. Here, we describe methods for visualizing nuclei using the phytochrome-derived autofluorescence in the root of Arabidopsis thaliana.
Materials and reagents
Plant materials
1. Arabidopsis thaliana (L.) Heynh. ecotype: Col-0
2. Arabidopsis thaliana transgenic plant harboring BOR1pro:BOR1-GFP [7]
Reagents
1. Ethyl alcohol (Sigma-Aldrich, catalog number: 09-0770)
2. Gellan gum (Fujifilm Wako, catalog number: 073-03075)
3. 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Roche, catalog number: 10236276001)
4. NaH2PO4·2H2O (Fujifilm Wako, catalog number: 192-02815)
5. NaHPO4·12H2O (Fujifilm Wako, catalog number: 196-02835)
6. MgSO4·7H2O (Fujifilm Wako, catalog number: 131-00405)
7. Ca(NO3)2·4H2O (Fujifilm Wako, catalog number: 039-00735)
8. KNO3 (Fujifilm Wako, catalog number: 160-04035)
9. MnSO4·H2O (Sigma, catalog number: M7634)
10. ZnSO4·7H2O (Fujifilm Wako, catalog number: 264-00402)
11. CuSO4·5H2O (Fujifilm Wako, catalog number: 039-04412)
12. CoCl2·6H2O (Fujifilm Wako, catalog number: 036-03682)
13. (NH4)6Mo7O24·4H2O (Fujifilm Wako, catalog number: 016-06902)
14. FeSO4·7H2O (Fujifilm Wako, catalog number: 098-01085)
15. Na2EDTA·2H2O (Sigma, catalog number: 345-01865)
16. Boric acid (Fujifilm Wako, catalog number: 026-15715)
17. Sucrose (Fujifilm Wako, catalog number: 196-00015)
Solutions
1. 200× Pi stock solution (see Recipes)
2. 200× Mg stock solution (see Recipes)
3. 200× Ca-K-N stock solution (see Recipes)
4. 200× Micronutrient (-B, -Fe) stock solution (see Recipes)
5. 200× Fe stock solution (see Recipes)
6. DAPI stock and staining solution (see Recipes)
7. MGRL media (see Recipes)
8. MGRL liquid medium without sugar (see Recipes)
Recipes
1. 200× Pi stock solution (pH 5.8)
NaH2PO4·2H2O 47.2 g/L
Na2H PO4·12H2O 18.4 g/L
This stock solution should be prepared using Milli-Q ultrapure water. Store in plastic bottles at room temperature.
2. 200× Mg stock solution
MgSO4·7H2O 74.0 g/L
This stock solution should be prepared using Milli-Q ultrapure water. Store in plastic bottles at room temperature.
3. 200× Ca-K-N stock solution
Ca(NO3)2·4H2O 94.4 g/L
KNO3 60.7 g/L
This stock solution should be prepared using Milli-Q ultrapure water. Store in plastic bottles at room temperature.
4. 200× Micronutrient (-B, -Fe) stock solution
MnSO4·H2O 348 mg/L
ZnSO4·7H2O 57.5 mg/L
CuSO4·5H2O 50 mg/L
CoCl2·6H2O 6.2 mg/L
(NH4)6Mo7O24·4H2O 5.9 mg/L
This stock solution should be prepared using Milli-Q ultrapure water. Store in plastic bottles at room temperature.
5. 200× Fe stock solution
FeSO4·7H2O 2.78 g/L
Na2EDTA·2H2O 3.72 g/L
This stock solution should be prepared using Milli-Q ultrapure water. Store in plastic bottles at room temperature.
6. DAPI stock and staining solution
Dissolve DAPI in ethanol at a concentration of 5 mg/mL (stock solution). For the staining solution, dilute DAPI stock solution 1,000-fold using MGRL liquid medium without sugar (final concentration of DAPI: 5 μg/mL).
7. MGRL media
1. Add the following reagents into ~700 mL of Milli-Q ultrapure water:
a. 200× Pi stock solution 4 mL
b. 200× Mg stock solution 4 mL
c. 200× Ca-K-N stock solution 4 mL
d. 200× Micronutrient stock solution 4 mL
e. 200× Fe stock solution 4 mL
2. Add 1 mM or 500 mM boric acid solution: For low-B medium, add 400 μL of 1 mM boric acid solution (0.5 μM B, final concentration). For normal-B medium, add 48 μL of 500 mM boric acid solution (30 μM B, final concentration).
3. Adjust the volume to 800 mL with Milli-Q ultrapure water.
4. Transfer the solution into a 1,000 mL NalgeneTM wide-mouth PPCO bottle.
5. Add 8 g of sucrose.
6. Add 12 g of gellan gum.
7. Autoclave at 121 °C for 15 min.
8. MGRL liquid medium without sugar
1. Add the following reagents into ~40 mL of Milli-Q ultrapure water in a 50 mL plastic conical tube.
a. 200× Pi stock solution 250 μL
b. 200× Mg stock solution 250 μL
c. 200× Ca-K-N stock solution 250 μL
d. 200× Micronutrient stock solution 250 μL
e. 200× Fe stock solution 250 μL
2. Add 1 mM or 500 mM boric acid solution: For low-B medium, add 25 μL of 1 mM boric acid solution (0.5 μM B, final concentration). For normal-B medium, add 3 μL of 500 mM boric acid solution (30 μM B, final concentration).
3. Adjust the volume to 50 mL with Milli-Q ultrapure water.
Laboratory supplies
1. Cover glass, 22 × 40 mm No. 1, 0.13–0.17 mm thickness (Matsunami Glass Ind., Ltd., catalog number: C022401)
2. 1.5 mL test tube
3. 1,000 μL pipette tip (used with 1,000 μL micropipette for washing seeds)
4. 200 μL pipette tip (used with 200 μL micropipette for sample mounting on slide glass)
5. 10 μL pipette tip (used with 20 μL micropipette for sowing)
6. 50 mL plastic conical tube
7. Sterile 25 mL serological pipette
8. Surgical tape 21N No. 12, 12 mm × 9 m (Nichiban Co., Ltd.)
9. Rectangle plastic dish, 140 mm × 100 mm × 14.5 mm (Eiken Chemical Co., Ltd., catalog number: AW2000)
10. Aluminum foil
11. Scalpel blade or razor blade
Equipment
1. 1,000 mL NalgeneTM wide-mouth PPCO bottles with closure (Nalgene Nunc, Thermo Fisher Scientific, catalog number: 312105-0032)
2. Autoclave
3. Plant growth chamber (NK System, catalog number: LH-411PFPFDT-SP)
4. Confocal laser scanning microscope equipped with 640 nm laser (Zeiss, model: LSM800)
5. Microscope objective lens, 40× water-immersion (1.1 NA LD C-Apochromat, Zeiss, item number: 421867-9970-000)
6. Microscope objective lens, 63× oil-immersion (1.4 NA Plan-Apochromat, Zeiss, item number: 420782-9900-000)
7. Slide glass (Matsunami Glass Ind., Ltd., catalog number: S012260)
8. Clean bench
9. 1,000 μL micropipette
10. 200 μL micropipette
11. 20 μL micropipette
12. Electric automatic pipette
Software and datasets
1. Fiji/ImageJ (ImageJ2, ver2.9.0/1.53t) (see https://imagej.net/software/fiji/) [8]
Procedure
文章信息
稿件历史记录
提交日期: Jan 6, 2025
接收日期: Mar 16, 2025
在线发布日期: Mar 25, 2025
出版日期: Apr 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yoshinari, A. and Nakamura, M. (2025). Near-Infrared Autofluorescence Imaging of Nuclei in Living Plant Roots. Bio-protocol 15(8): e5274. DOI: 10.21769/BioProtoc.5274.
分类
植物科学 > 植物细胞生物学 > 细胞成像
细胞生物学 > 细胞成像 > 共聚焦显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link