- EN - English
- CN - 中文
Visualization of F-Actin Through Expansion Microscopy (ExM) with Trifunctional Linker-Conjugated Phalloidin
利用三功能连接子标记的鬼笔环肽结合扩增显微术可视化F-Actin
发布: 2025年04月20日第15卷第8期 DOI: 10.21769/BioProtoc.5273 浏览次数: 2207
评审: Tadamoto IsogaiMunenori IshibashiTejeshwar Rao
Abstract
Expansion microscopy (ExM) is an imaging technique that enables super-resolution imaging of biological specimens using conventional confocal microscopy. This process entails the isotropic physical expansion of a (biomolecular) sample that has been cross-linked to a swellable polymer. The grafting of biomolecules (and the subsequent fluorescent readout) is accomplished by introducing an acryloyl group to the amine groups of lysine residues within the proteins, enabling subsequent imaging. However, visualizing actin filaments with high spatial resolution using ExM remains challenging. Herein, we report the construction of a phalloidin conjugate containing actin stains and their application in ExM. This protocol highlights the efficacy of trifunctional linker (TRITON/Actin-ExM) for F-actin imaging, demonstrating that TRITON-labeled actin allows for efficient anchoring and signal retention, enabling robust visualization of actin filaments in expansion microscopy.
Key features
• Engineered linker (TRITON) design ensures efficient fluorophore attachment, resulting in bright, stable signals during imaging.
• Performed pre-expansion and antibody-free labeling.
• Detailed and specific visualization of actin filaments in ExM experiments (4-fold expansion).
Keywords: Trifunctional phalloidin conjugates (三功能鬼笔环肽)Graphical overview
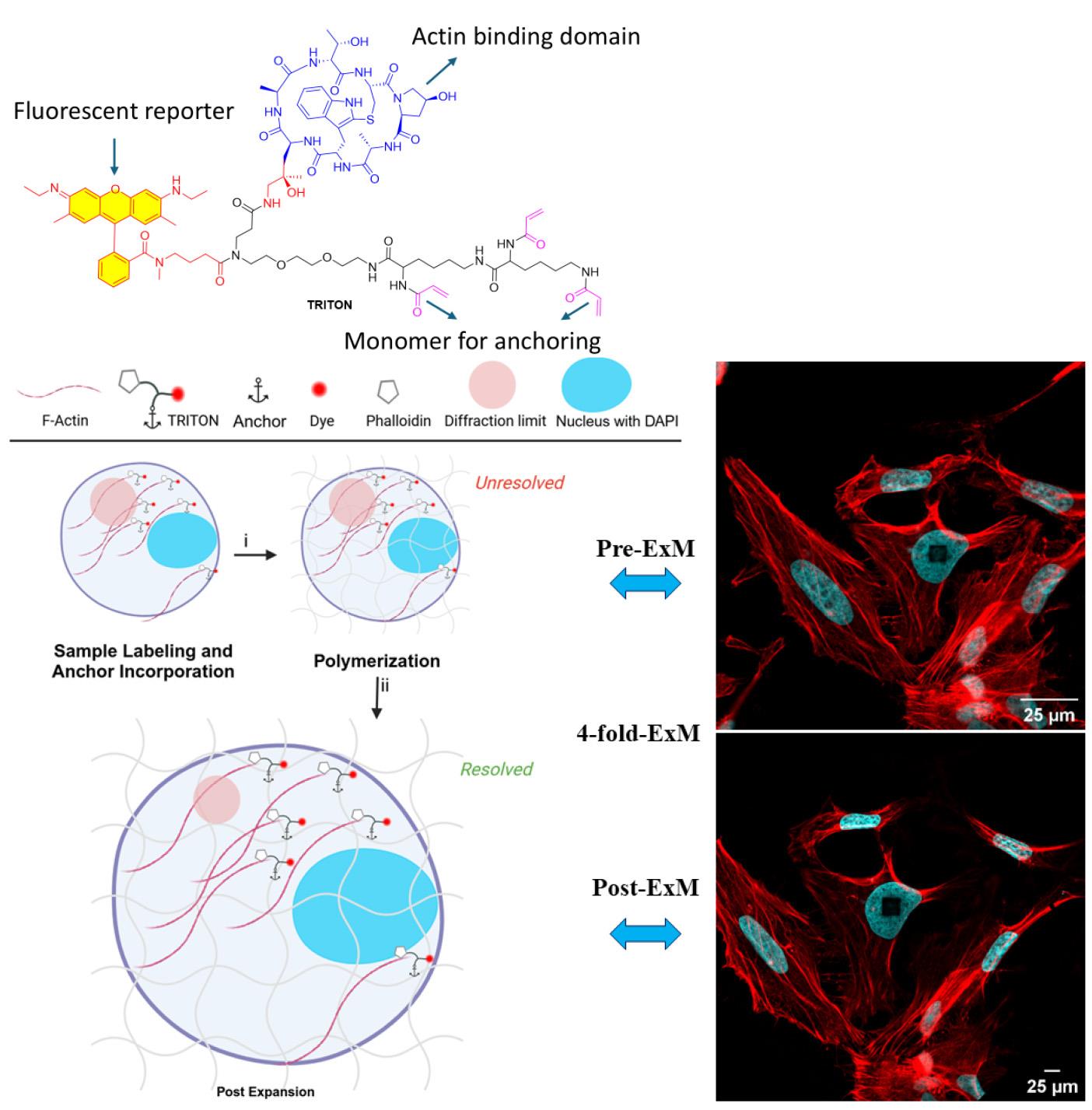
Background
Expansion microscopy (ExM) has emerged as a promising alternative technique for achieving super-resolution, offering nanoscale insights into biological samples without using dedicated super-resolution microscopy techniques. Boyden and colleagues first introduced ExM in 2015 [1], enabling confocal microscopes to achieve an effective resolution of approximately 70 nm. Since its introduction, ExM has seen substantial advancements, with efforts focused on broadening its range of applications and enhancing the achievable resolution [2–11]. In the ExM approach, rather than circumventing the diffraction limit, microscopes are employed to image physically isotropically expanded samples. Biological specimens are first embedded in a swellable polyelectrolyte hydrogel, followed by a homogenization step to eliminate structures that could impede uniform expansion. Homogenization is typically achieved via enzymatic digestion, such as with proteinase K, or through chemical denaturation by applying elevated temperatures or detergent treatments. The subsequent swelling of the hydrogel in Milli-Q (MQ) water increases the physical separation between labeled biomolecules, thereby enhancing spatial resolution. However, visualizing actin filaments using ExM remains a challenge.
Actin, one of the most abundant proteins in eukaryotic cells, plays a pivotal role in various cellular processes, including the regulation of cell motility [12], maintenance of cell morphology [13], cell division, transcriptional regulation [14], endocytosis [15], and so forth [16]. Functionalized phalloidin derivatives offer a widely employed fluorescent stain for actin filaments; however, this approach meets significant challenges within the framework of ExM, as the signal fails to graft to the hydrogel [8,17]. Consequently, improved approaches for visualizing actin filaments through ExM have attracted considerable interest [8,18,19].
To address the issue of poor grafting of actin stains in ExM, three distinct strategies for anchoring phalloidin-actin complexes have been reported [7,8]. The first involves synthesizing a trivalent phalloidin linker, called TRITON, which incorporates rhodamine B, phalloidin, and an acrylate monomer. This engineered design enables gel grafting, facilitating the post-expansion visualization of actin filaments [6,8,20]. The second strategy, proposed by Park et al., employs antifluorophore antibodies that bind to the fluorophore of phalloidin conjugates, enabling ExM imaging of actin filament organization using the original ExM protocol [18]. The third strategy, developed by Trinks and colleagues [19], uses a biotinylated phalloidin conjugate (phall-XX-biotin) followed by fluorescent streptavidin conjugation, where streptavidin introduces polymerizable moieties through the addition of lysine residues, enabling gel anchoring. In the latter two strategies, however, the large sizes of antibodies (~150 kDa) and streptavidin (~60 kDa) compared to TRITON (<2 kDa) cause linkage errors of 17.5 nm and 5 nm [21–23], respectively, which are exacerbated after expansion. Moreover, the bulkiness of antibodies and streptavidin hampers the effective binding of actin-bound phalloidin conjugates, resulting in their removal post-expansion and reduced fluorescence intensity, thus limiting the applicability of 4X ExM. Conversely, trivalent phalloidin linkers minimize linkage error, and their high gel-grafting efficiency leads to optimal imaging. As such, the protocol described here has recently been demonstrated to provide superior actin staining performance. Linear triacrylamide units are polymerized through free radical–induced polymerization, where free radicals initiate the formation of long-chain polymers by reacting with triacylamide monomers. This process enables ultrastructural imaging of actin across various expansion microscopy protocols and imaging modalities, and the method is rapidly gaining adoption in the field.
Materials and reagents
Biological materials
1. Mammalian cells in culture: HeLa (ATCC® CCL-2TM)
Reagents
1. DMEM, high glucose, no glutamine, no phenol red (Gibco, catalog number: 31053044), stored at 4 °C
2. Fetal bovine serum (FBS) (Sigma, catalog number: F7524), stored at -20 °C until use
3. GlutaMAX I, 200 mM (Gibco, catalog number: 35050038), stored at 4 °C
4. Gentamicin (50 mg/mL) (Gibco, catalog number: 15750037), stored at -20 °C until use
5. Dulbecco's phosphate-buffered saline (DPBS), without calcium and magnesium (Gibco, catalog number: 14190250), stored at 4 °C
6. Trypsin-EDTA 0.5%, no phenol red (Gibco, catalog number: 15400054), stored at 4 °C
7. Trypan blue stain 0.4% (Gibco, catalog number: 15250061), stored at 4 °C
8. Pierce 16% formaldehyde (w/v), methanol-free (Thermo Scientific, catalog number: 28908), stored at room temperature (RT)
9. Tris(hydroxymethyl)aminomethane (Tris) (Sigma, catalog number: 252859), stored at RT
10. Sodium acrylate 97% (Sigma-Aldrich, catalog number: 408220), stored at RT inside a desiccator
11. Acryloyl-X SE, >95.0% (AcX) (TCI America, catalog number: A345025MG), stored at -20 °C
12. Guanidine hydrochloride 99.5% (Thermo Scientific, catalog number: 364791000), stored at RT
13. Poly-L-lysine solution 0.1% (w/v) in H2O (Sigma-Aldrich, catalog number: P8920), stored at RT
14. Triton X-100 98%, DNAse free, RNAse free, protease free (Thermo Scientific, catalog number: 327371000) stored at 4 °C
15. Sigmacote (Sigma-Aldrich, catalog number: SLCM2185), siliconizing reagent for providing hydrophobic properties and reducing nonspecific adsorption; stored at 4 °C
16. 4-Hydroxy-TEMPO 97% (Sigma-Aldrich, catalog number: 176141), stored at RT
17. Proteinase K (New England Biolabs, catalog number: P81075), stored at -20 °C
18. Ethylenediaminetetraacetic acid (EDTA), 0.5 M) (Thermo Scientific, catalog number: J15694-AE) stored at RT
19. DAPI (4',6-diamidino-2-phenylindole, dihydrochloride, 10 μM in DMSO) (Invitrogen, catalog number: D1306) stored at -20 °C
20. Fluorescent phalloidin ExM reagent (TRITON); synthesized in-house according to [8] or commercially available as Actin ExM 532 (Chrometra Scientific, catalog number: ER-10-4-S), stored at -20 °C until use
21. Phosphate-buffered saline (PBS) 1× pH 7.4, without calcium, magnesium, and phenol red (Gibco, catalog number: 10010023)
22. Phosphate-buffered saline (PBS) 10× pH 7.4, without calcium, magnesium, and phenol red (Gibco, catalog number: P7059)
23. Ammonium persulfate (APS) (Sigma-Aldrich, catalog number: A3678)
24. Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: 213330)
25. Hydrochloric acid 37% (HCl) (Sigma-Aldrich, catalog number: 258148)
26. N,N,N',N'-Tetramethyl ethylenediamine (TEMED) (Sigma-Aldrich, catalog number: T7024)
27. N,N'-Methylenebisacrylamide (Sigma-Aldrich, catalog number: 146072)
28. Acrylamide (Sigma-Aldrich, catalog number: A8887)
29. Sodium chloride (Sigma-Aldrich, catalog number: S9888)
30. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650)
Solutions
1. Gelation stock (polymerization, 10 mL) (see Recipes)
2. 4-hydroxy-TEMPO stock solution (see Recipes)
3. Catalyst (see Recipes)
4. Active monomer solution (200 μL) (see Recipes)
5. Digestion solution (see Recipes)
6. AcX (acryloyl-X) (see Recipes)
7. 1 M HCl solution (see Recipes)
8. 100 mM NH4Cl solution (see Recipes)
9. 5 M NaCl solution (100 mL) (see Recipes)
10. 4 M Guanidine HCl (100 mL) (see Recipes)
11. 1 M Tris, pH 8 (100 mL) (see Recipes)
Recipes
General note: Some reagents, such as those involved in polymerization or gelation steps, should be prepared just before use to ensure optimal activity and avoid degradation.
1. Gelation stock (polymerization, 10 mL)
| Reagent | Stock concentration | Quantity or Volume | Final concentration |
|---|---|---|---|
| Sodium acrylate | 3.4 g/10 mL MQ | 2.525 mL | 8.6 g/100 mL |
| Acrylamide | 5 g/15 mL MQ | 0.75 mL | 2.5 g/100 mL |
| N,N'-methylenebisacrylamide | 0.2 g/15 mL MQ | 1.125 mL | 0.15 g/100 mL |
| Sodium chloride (Recipe 9) | 5 M (in MQ) | 4 mL | 2 M |
| PBS | 10× | 1 mL | 1× |
| MQ water | 0.6 mL | ||
| Total | 10 mL |
Note: Prepare aliquots of 188 μL (polymerization for one sample) and store at -20 °C.
2. 4-hydroxy-TEMPO stock solution
0.5 g per 100 mL of MQ water.
Prepare aliquots and store at -20 °C.
3. Catalyst
| Reagent | Quantity or Volume | Final concentration |
|---|---|---|
| APS | 0.1 g | 10% (w/v) |
| MQ water | 0.95 mL | |
| Total | 1 mL |
Prepare aliquots and store at -20 °C.
| Reagent | Quantity or Volume | Final concentration |
|---|---|---|
| TEMED | 12.9 μL | 10% (v/v) |
| MQ water | 987.1 μL | |
| Total | 1 mL |
Prepare aliquots and store at -20 °C.
4. Active monomer solution (200 μL)
| Reagent | Quantity or Volume | Final concentration |
|---|---|---|
| Gelation stock (Recipe 1) | 188 μL | 0.15% |
| 4-Hydroxy-TEMPO (Recipe 2) | 2 μL | 0.5% |
| APS | 4 μL | 10% |
| TEMED | 4 μL | 10% |
| MQ water | 2 μL | |
| Total | 200 μL |
Note: Keep the solution on ice until ready to use. Add APS and TEMED (see Recipe 3) just before use.
5. Digestion solution
| Component | Stock concentration | Quantity or Volume | Final concentration |
|---|---|---|---|
| Tris | 1 M, pH 8 (in MQ) | 2.5 mL | 0.05 M |
| EDTA | 0.5 M | 0.1 mL | 0.001 M |
| Triton X-100 | 100% | 0.25 mL | 0.5% (v/v) |
| Guanidine HCl | 4 M (in MQ) | 10 mL | 0.8 M |
| MQ water | 37.15 mL | ||
| Total | 50 mL |
Note: The 1 M stock solution of Tris was adjusted to pH 8 using 37% HCl.
6. AcX (acryloyl-X)
a. To prepare AcX stock solutions, dissolve AcX in anhydrous DMSO to a concentration of 10 mg/mL and store 10 μL aliquots at -20 °C.
b. Thaw the 10 mg/mL AcX stock to RT and dilute it 1:100 to a final concentration of 0.1 mg/mL by adding 990 μL of 1× PBS.
7. 1 M HCl solution
To prepare the desired concentration of HCl from the stock solution (typically 37%), dilute it by mixing 8.3 mL of concentrated HCl with 91.7 mL of MQ water.
8. 100 mM NH4Cl solution
a. Measure 0.534 g of NH4Cl.
b. Dissolve it in approximately 90 mL of PBS.
c. Adjust the final volume to 100 mL with PBS.
d. Store at 4 °C for short-term use or aliquot and freeze for long-term storage.
9. 5 M NaCl solution (100 mL)
a. Weigh 29.2 g of NaCl using an analytical balance.
b. Add the NaCl to approximately 80–90 mL of MQ water in a volumetric flask.
c. Once the NaCl has fully dissolved, add MQ water to bring the final volume to 100 mL.
d. Stir the solution thoroughly to ensure uniformity.
e. Store the 5 M NaCl solution at room temperature
10. 4 M guanidine HCl (100 mL)
a. Measure 38.212 g of guanidine HCl using an analytical balance.
b. Dissolve it in approximately 90 mL of MQ water in a volumetric flask.
c. Adjust the final volume by adding MQ water to 100 mL.
d. Mix the solution thoroughly and store the 4 M guanidine HCl solution a 4 °C.
11. 1 M Tris (pH 8) (100 mL)
a. Weigh out 12.1 g of Tris base using an analytical balance.
b. Add 80–90 mL of MQ water to dissolve it and stir the solution until the Tris base is completely dissolved.
c. Slowly add 1 M HCl (Recipe 7) to adjust the pH to exactly 8.0. Add the HCl drop by drop, stirring continuously to avoid overshooting the pH.
d. After adjusting the pH, transfer the solution to a 100 mL volumetric flask.
e. Swirl the solution gently to ensure it is homogeneous and store the 1 M Tris solution at 4 °C.
Laboratory supplies
1. T75 flask for cell culture (Thermo Fisher Scientific, catalog number: 156800)
2. 22 × 22 mm, #1.5H coverslips (https://www.carlroth.com/be/en/coverslips/coverslips-thickness-1-5/p/kcy1.1)
3. Microscope slides 26 × 76 mm, single concave (see Figure S2) (Pearl, catalog number: 7103)
4. Microscope slides 26 × 76 mm (https://www.galvoptics.co.uk/optical-components/microscope-slides/microscope-slides/?utm_source=google&utm_medium=cpc&utm_campaign=Galvoptics&utm_content)
5. 6-well plates (Thermo Fisher Scientific, catalog number: 150239)
6. Dumont HP crossover tweezers N7, Dumoxel 0.17 × 0.10 mm tip (Dumont, catalog number: AGT5007)
7. Microcentrifuge tubes (Eppendorf, catalog numbers: 0030121023, 0030120086)
8. Conical tube 15 mL/50 mL (Greiner Bio-One, catalog number: 188271/227261)
9. Cytosmart counting chamber with cover glass (Cytosmart, Corning, catalog number: 0699910591)
10. P1000 regular pipette tips (Thermo Scientific Finnpipette, catalog number: 94060717)
11. P200 regular pipette tips (Thermo Scientific Finnpipette, catalog number: 94060317)
12. P10 regular pipette tips (Thermo Scientific Finnpipette, catalog number: 94060117)
13. Spatula (Bochem, catalog number: 3101)
14. Parafilm (Merck, catalog number: P7793)
15. Scissors
16. 500 mL beaker
17. Photo glue (Collall, catalog number: COLFO0100)
18. 6-well glass bottom plate (confocal imaging dish) (see Figure S6) (Cellvis, catalog number: P06-1.5H-N)
Equipment
1. 37 °C incubator with 5% CO2 in a humidified environment (Thermo Scientific, model: HERACELL VIOS 160i)
2. Cytosmart cell counter (Corning, catalog number: 6749)
3. Incubator INCU-Line® IL 10 (VWR, catalog number: 390-0384)
4. Microscope (Leica Microsystems GmbH, model: TCS SP8)
5. Objective lenses HC PL APO CS2 63×/1.20 WATER and HC PL APO CS2 20×/0.75 DRY
Software and datasets
1. Fiji (ImageJ, https://imagej.net/software/fiji/) [24]
2. LAS X (https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/)
3. BioRender (https://www.biorender.com/) was used for the Graphical overview: https://app.biorender.com/illustrations/66d5e6f134b972cb4b0abed5?slideId=505c1415-eac1-400b-83fe-dd8171f5f3d3
Procedure
文章信息
稿件历史记录
提交日期: Oct 22, 2024
接收日期: Mar 2, 2025
在线发布日期: Mar 27, 2025
出版日期: Apr 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Huang, J., Wen, G., Iven, T., Linhares, D., Koo, L., Sauer, M., Dehaen, W., Leen, V. and Hofkens, J. (2025). Visualization of F-Actin Through Expansion Microscopy (ExM) with Trifunctional Linker-Conjugated Phalloidin. Bio-protocol 15(8): e5273. DOI: 10.21769/BioProtoc.5273.
分类
细胞生物学 > 细胞成像 > 超分辨率成像
生物化学 > 蛋白质 > 成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













