- EN - English
- CN - 中文
The Mouse Social Frailty Index (mSFI): A Standardized Protocol
小鼠社会虚弱指数(mSFI):标准化实验方案
发布: 2025年04月20日第15卷第8期 DOI: 10.21769/BioProtoc.5272 浏览次数: 1820
评审: Marco Pagliusi Jr.Anonymous reviewer(s)
Abstract
The advent of geroscience engendered the development of approaches to quantify the aging process and estimate biological age on an individual level. Recognizing that declines observed in aging are not only physical but also social led us to develop a mouse Social Frailty Index (mSFI) designed to quantify age-related impairments of social functioning in mice. The mSFI consists of seven behavioral assays that measure essential facets of social behavioral functioning in mice: social communication, social interaction, and social functional ability. The assays that comprise the mSFI are all minimally disruptive, relatively simple to execute, and optimized for compatibility with longitudinal studies utilizing experimental interventions relevant to geroscience. The mSFI is conducted over AM and PM sessions spanning a maximum of 3.5 days, using materials common to most animal facilities. The data for all assays is obtained observationally, manually recorded, and entered into predefined template sheets that automate the computation of the mSFI. We have demonstrated the validity and applicability of the mSFI across multiple laboratory sites and experiments. This index has proven to discriminate between differential trajectories of biological aging driven by sex, progeria, or social stress-relevant contexts. The mSFI represents a novel index to quantify trajectories of biological aging in mice, and its application may help elucidate the social dimensions of the aging process.
Key features
• The mSFI is a comprehensive assessment for the evaluation of impairment in social behavioral functioning related to aging in mice.
• Minimally disruptive, relatively simple, commonly used high-throughput assays of spontaneous social behavior that are optimized for compatibility with longitudinal studies of aging.
• The protocol spans AM and PM sessions over 3.5 days maximum; the sequence of individual assays is flexible by design.
• The mSFI requires materials common to most animal research facilities.
• mSFI application is compatible with most experimental treatments, social behavioral paradigms, longitudinal within-subject designs, and genetically modified mouse models.
Keywords: Social frailty (社会虚弱)Graphical overview
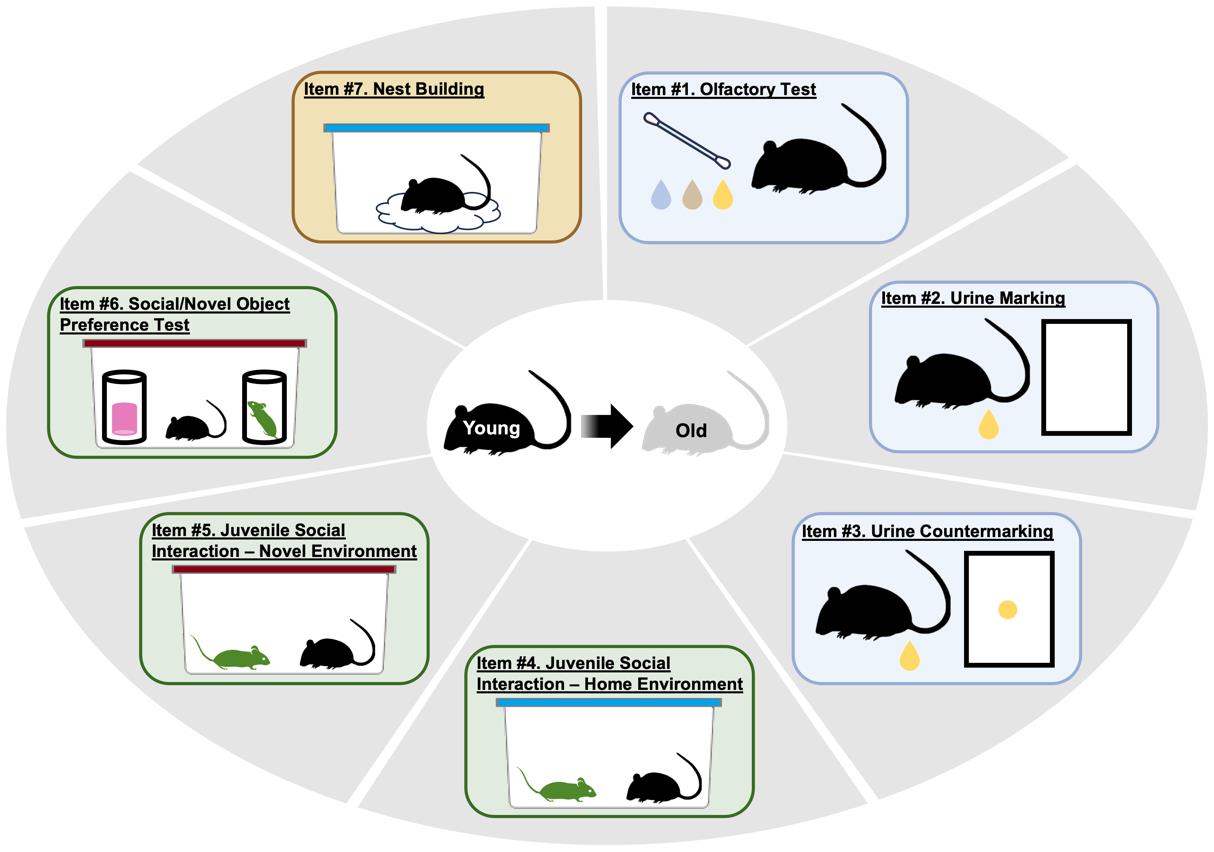
The mouse social frailty index (mSFI)
Background
As a consequence of societal advances allowing modern humans to live longer than ever before, aging now represents the single largest risk factor for chronic diseases and mortality [1–4]. Although the aging process is characterized by gradual and progressive decline, individuals of the same chronological age may manifest a different biological age, with some experiencing a variety of age-related morbidities while others remain relatively healthy [5–10]. Given this marked heterogeneity in the aging process, it is imperative that the growing field of geroscience be equipped with measures that reliably capture the quality of biological aging on an individual level in order to better understand and leverage mechanisms underlying the basic biology of aging to lengthen healthspan [11–17]. Frailty, an age-related state of increased vulnerability to adverse health outcomes, has emerged as a reliable and valid outcome in the quantification of functional impairment and biological aging [18–21]. Indices based on an accumulation of physical health deficits over time have been constructed for both humans and mice, demonstrating high evolutionarily conserved predictive validity for age-related morbidity and mortality while providing high utility as a robust indicator of biological aging for clinical practice and preclinical research [22–30].
However, many of the factors that produce disparities in healthspan and dictate the pace of biological aging across species are socially bound and linked to social determinants [31–37]. In this light, social frailty, an age-related lack of social skills and resources necessary to fulfill basic social needs and achieve well-being, has been proposed as a construct and measured in humans, showing predictive validity for mortality [38,39]. Despite the widespread use of mice as an animal model for geroscience, and studies demonstrating evolutionarily conserved declines in their social capabilities with age, no extant method has been able to quantify the social aspects of frailty in laboratory mice [40–43]. To fill this gap, we recently developed a novel approach to quantify social frailty in mice [44]. The mouse Social Frailty Index (mSFI) is based on the deficit accumulation model of frailty [20,22,45] and consists of seven minimally disruptive, relatively simple, commonly used assays that measure essential facets of social behavioral functioning in mice: social communication, social interaction, and social functional ability. The mSFI is conducted over a maximum of 3.5 days, in AM and PM sessions. During each session, each mouse is tested from a few minutes to one hour in duration (unless otherwise specified). Behavioral data are either directly recorded during testing or quantified later in assays that are based on image quantification via freely available software. The obtained values are then entered into predefined spreadsheet templates that automate the calculation of the mSFI. In practice [44], the mSFI showed a progressive, graded increase with chronological age in group-housed C57BL6 mice as well as sensitivity to i) sex, ii) social isolation and chronic subordination stress, and iii) mouse models of progeria. Our published data [44] provide robust support for the initial validity and applicability of the mSFI as a useful measure for preclinical geroscience research. This protocol describes and operationalizes the procedure for conducting the mSFI.
Materials and reagents
Biological materials
1. Juvenile (4–5 weeks old) stimulus mice matched to the sex and strain of experimental mice (see General Note 1). Testing can be conducted with up to five mice per observer, per round of observation.
a. Juvenile mice can be reused in subsequent rounds of observation, so the amount required is one that is sufficient to provide one round of experimental mice with sex- and strain-matched juveniles. For example, 5 juvenile mice could be used for 20 experimental mice. This would result in four rounds of observation.
b. If juvenile mice are of the same coat color as the experimental mouse, mark the tail of the juveniles with a bright-colored marker for ease of observation and to differentiate between juveniles themselves.
Reagents
1. Tap water
2. 1:10,000 pure vanilla extract (McCormick & Co., Inc., Hunt Valley, MD) diluted in tap water
3. Pooled intact male & female mouse urine purchased from a commercial vendor (Biochemed Pharmacologicals, catalog number: NC1569592), ~2.2 mL/20 mice as a social odor (see also General Notes 1 and 2)
4. 70% EtOH spray
Laboratory supplies
1. Small static cages (186 mm × 298 mm × 128 mm) without bedding, nesting material, food, or water bottle
2. Small static cages (186 mm × 298 mm × 128 mm) with a layer of bedding but without nesting material, food, or water bottle
3. Large static cages (257 mm × 483 mm × 152 mm) with bedding but without nesting material, food, water bottle, or metal wire lids
4. Wire mesh cups (e.g., Amazon, Amazon Basics wire mesh pen cup, reference: B07VVB7DSK)
5. Bottles filled with tap water (e.g., EMD Milipore Corporation, Milipore Express® PLUS 0.22 mm PES 150 mL bottle unit) or any other available object to serve as a weight on top of the wire mesh cups to prevent the mouse from tipping them while exploring
6. Novel objects (e.g., Simport Scientific Inc., catalog number: C576, SecurTainerTM II tamper-evident container, 40 mL, reference: C576-40MA). These may be changed based on available resources but must fit in the wire mesh cups and be similar in size to a juvenile mouse
7. Pressed cotton batting nestlets (e.g., Ancare Corp, reference: NES3600)
8. GeneMate 20–200 μL autoclavable single-channel pipettor (ISC BioExpress Inc. or an equivalent model) and pipette tips
9. 15.24 cm cotton swabs (e.g., Tyco Healthcare Group LP, Kendall CurityTM single tipped applicators, reference: 8884540500)
10. 15 mL polystyrene conical tubes (e.g., Corning, Falcon® 15 mL polystyrene centrifuge tube, catalog number: 352095)
11. Tube rack (recommended) (e.g., Heathrow Scientific LLC, 5.39 cm wd, 9.52 cm ht, 17.46 cm lg, polypropylene, Grainger, catalog number: 55PT41)
12. 31.5 × 33.5 cm Whatman 3 MM CHR chromatography blotting paper (Cytiva, catalog number: 3030-335)
13. One stopwatch per experimenter + one additional
14. One clipboard per experimenter
15. Coding sheets for instantaneous sampling
16. Optional: sticky notes to keep track of mouse IDs
17. If needed: cages to temporarily store cage mates or house overnight for nest building
Equipment
1. UV transilluminator (e.g., Ultra-Violet Products, Inc., model: chromato-vue transilluminator 0–62)
2. High-resolution camera (high-resolution phone camera is OK; you must ensure that either the same camera is used across assessments or that megapixel count is identical between cameras used)
Software and datasets
1. Microsoft Excel or any other spreadsheet-based software
2. ImageJ (Version 1.53m [46])
3. Statistical software of your choosing to produce data in a reproducible manner, adequate for the comparisons you are making, based on your experimental design
Procedure
文章信息
稿件历史记录
提交日期: Dec 4, 2024
接收日期: Mar 11, 2025
在线发布日期: Mar 25, 2025
出版日期: Apr 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Collinge, C. W., Razzoli, M. and Bartolomucci, A. (2025). The Mouse Social Frailty Index (mSFI): A Standardized Protocol. Bio-protocol 15(8): e5272. DOI: 10.21769/BioProtoc.5272.
分类
神经科学 > 行为神经科学 > 认知
神经科学 > 行为神经科学 > 实验动物模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













