- EN - English
- CN - 中文
Optimizing Confocal Imaging Protocols for Muscle Fiber Typing in the Mouse Masseter Muscle
小鼠咬肌肌纤维类型分析的共聚焦成像方案优化
发布: 2025年04月05日第15卷第7期 DOI: 10.21769/BioProtoc.5267 浏览次数: 2916
评审: Sreejith PerinthottathilElena A. OstrakhovitchAnonymous reviewer(s)

相关实验方案

基于Fiji ImageJ的全自动化流程开发:批量分析共聚焦图像数据并量化蛋白共定位的Manders系数
Vikram Aditya [...] Wei Yue
2025年04月05日 2879 阅读
Abstract
The masseter muscle, a key orofacial muscle, demonstrates unique anatomical and functional properties, including sexual dimorphism in myosin heavy chain (MyHC) expression and complex fiber architecture. Despite its importance in mastication and relevance to various disorders, phenotypic characterization of the masseter remains limited. Conventional fluorescence microscopy has been a cornerstone in muscle fiber typing, reliably identifying MyHC isoforms and measuring fiber cross-sectional areas. Building on this foundation, confocal microscopy offers complementary advantages, such as enhanced resolution, increased flexibility for multiplexing, and the ability to visualize complex structures in three dimensions. This study presents a detailed protocol for using confocal microscopy to achieve high-resolution imaging and molecular characterization of masseter muscle cryosections. By leveraging advanced technologies such as white light lasers and extended z-length imaging, this method ensures precise spectral separation, simultaneous multichannel fluorescence detection, and the ability to capture muscle architecture in three dimensions. The protocol includes tissue preparation, immunostaining for MyHC isoforms, and postprocessing for fiber segmentation and quantification. The imaging setup was optimized for minimizing signal bleed through, improving the signal-to-noise ratio, and enabling detailed visualization of muscle fibers and molecular markers. Image postprocessing allows for quantification of the cross-sectional area of individual fibers, nuclei location measurements, and identification of MyHC isoforms within each fiber. This confocal microscopy–based protocol provides similar resolution and contrast compared to conventional techniques, enabling robust multiplexed imaging and 3D reconstruction of muscle structures. These advantages make it a valuable tool for studying complex muscle architecture, offering broad applications in muscle physiology and pathology research.
Key features
• Enables high-resolution imaging of muscle fiber architecture, capturing detailed spatial relationships using extended z-length and advanced spectral separation techniques.
• Supports simultaneous detection of multiple molecular markers for robust muscle fiber typing and molecular localization.
• Allows for the generation of three-dimensional models to analyze muscle structures such as neuromuscular junctions, extracellular matrix, and mitochondrial organization.
• Adaptable to various skeletal muscles and species, providing valuable insights into muscle physiology, regeneration, and disease processes.
Keywords: Muscle fiber typing (肌纤维分型)Graphical overview
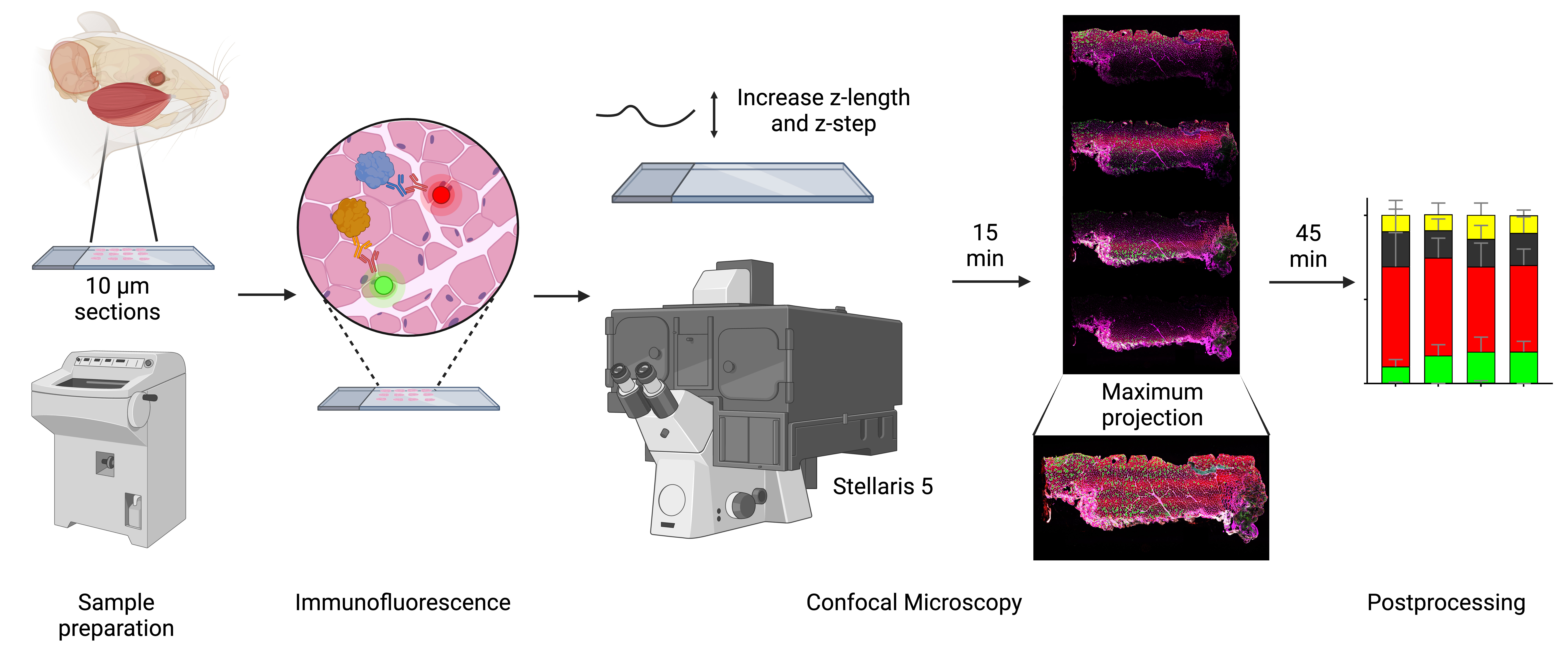
Analyzing muscle fiber composition and morphology in mice’s masseter muscle using confocal microscopy. Workflow for characterizing rodent masseter muscle fibers using advanced confocal microscopy. Confocal microscopy, equipped with white light laser technology and optimized z-stack imaging, allows precise spectral unmixing to reduce bleed through and enhance signal detection. The z-length is extended beyond the physical thickness of the sample to account for potential variations in tissue flatness and ensure complete imaging of all focal planes. The resulting high-resolution images provide detailed insights into fiber architecture, molecular composition, and cross-sectional areas, ensuring robust and reproducible data for analyzing the complex phenotypic characteristics of the masseter and other muscles.
Background
Skeletal muscles are heterogeneous, allowing them to engage in a spectrum of activities, from continuous low-intensity activity, such as breathing and posture control, to repeated submaximal or maximal contractions required for sprinting or lifting heavy objects [1]. Historically, muscle fibers have been categorized based on their morphological (red vs. white), biochemical (oxidative capacity, as determined by measuring succinate dehydrogenase, nicotinamide adenine dinucleotide dehydrogenase reductase, or ATP synthase activities), or molecular characteristics [2–6]. Currently, fiber types are distinguished based on the expression of specific myosin heavy chain (MyHC) isoforms. In adult rodent muscles, four major isoforms are detected, including type 1, 2A, 2X, and 2B (or a combination of these in hybrid fibers) corresponding to MYH7, MYH2, MYH1, and MYH4, respectively [1,3,7,8]. Type 1 fibers (slow twitch) have high mitochondrial content, oxidative metabolism, and slow contraction speed, making them well-suited for sustained endurance activities. Type 2A fibers (fast twitch oxidative) can generate greater power while maintaining endurance capacity. Type 2X and type 2B fibers (fast twitch glycolytic) contract the fastest and are adapted for short bursts of power. Noticeably, metabolic and contractile properties are highly adaptable and context-dependent, influenced by muscle function, species, and environmental factors rather than strictly by MyHC expression alone [3].
The orofacial muscles constitute a subset of skeletal muscle originating from the branchial arches, a distinct origin from muscles of the trunk and limbs [9]. The masseter is the dominant jaw-closing muscle in rodents, accounting for approximately 50% of the masticatory muscle mass and contributing to mastication, biting, and swallowing [10]. The adult muscle masseter consists of three anatomical partitions: superficial, intermediate, and deep [10–12]. Fiber orientation shifts from vertical in the anterior regions to an oblique orientation in the posterior aspect of the superficial and intermediate layers [10]. Notably, the insertion area for the superficial and deep masseter is vast, extending from the mandible angle to the first molar position for the superficial masseter and almost the entire length of the jugal bone for the deep masseter [10]. Given the complex architecture of the masseter, high-resolution imaging techniques are essential to capture its detailed structure [13]. However, the phenotypic characterization of the masseter remains limited [14,15].
Determining MyHC isoforms by immunofluorescence for muscle fiber typing has become widespread, in part because it also provides information about fiber cross-sectional areas [1,2,16]. Further, simultaneous labeling of muscle fibers is possible thanks to mouse monoclonal anti-MyHC antibodies with isotype-specific secondary antibodies [17]. Fluorescence microscopy often uses optical filters to separate emission from excitation light, accommodating several filter cubes, each one directing a specific predetermined wavelength [18]. Although only a single filter cube may be engaged at a time, there is potential for spectral overlap between fluorophores, limiting the number of compatible markers in a single experiment. Additionally, fluorescence microscopy captures light from all focal planes, limiting the signal-to-noise ratio and image resolution [19].
To overcome the limitations of widefield microscopy, Marvin Minsky developed the confocal microscope in 1957 [20]. Confocal microscopy provides better resolution than widefield fluorescent microscopes, yet its main advantage is the ability to acquire high-contrast images by minimizing out-of-focus light using a pinhole [18,21]. Additionally, confocal microscopy can capture multiple optical planes, making it ideal for analyzing compartmentalization and colocalization of specific molecules with high accuracy, as well as enabling the creation of 3D reconstruction of the imaged tissue [21]. Newer systems, such as the Stellaris 5 (Leica Microsystems, Durham, NC), feature next-generation white light lasers (WLL), which allow for the simultaneous excitation and detection of multiple fluorophores. The fine-tuning of excitation wavelengths enables highly efficient spectral unmixing, overcoming the limitations of traditional systems. This technology expands the range of usable fluorophores, including those in the near-infrared spectrum, and supports the use of multiple labels in parallel [22,23].
The objective of this study is to demonstrate the effective use of confocal microscopy in obtaining high-resolution, quantifiable images for muscle fiber typing from cryosections. We aim to show that this approach offers excellent resolution and contrast, even in applications where confocal capabilities are typically deemed unnecessary. Multiple fluorescent channels allow for the simultaneous visualization of several molecular markers in addition to the corresponding MyHC labels. This flexibility provides detailed insights into muscle fiber architecture and is particularly advantageous for reducing background noise and imaging alongside other protein targets that require precise 3D localization.
Materials and reagents
Biological materials
1. Adult female triple transgenic 3x-Tg-AD mice (Mutant Mouse Resource and Research Center, National Institutes of Health). Mice were 6–7 months old at tissue harvest. At 4 months of age, female 3xTg-AD mice show early signs of amyloid-beta plaques and tau phosphorylation, while behavioral phenotypes remain subtle and task-dependent [24–27]
2. Primary antibodies
a. Anti-laminin antibody (MilliporeSigma, catalog number: L9393, lot 0000234279, 0.5 mg/mL)
b. Anti-myosin heavy chain slow (Developmental Studies Hybridoma Bank–DSHB, catalog number: BA-F8, supernatant)
c. Anti-myosin heavy chain 2A (Developmental Studies Hybridoma Bank–DSHB, catalog number: SC-71, supernatant)
d. Anti-myosin heavy chain 2X (Developmental Studies Hybridoma Bank–DSHB, catalog number: 6H1, supernatant)
e. Anti-myosin heavy chain 2B (Developmental Studies Hybridoma Bank–DSHB, catalog number: BF-F3, supernatant)
3. Secondary antibodies
a. Goat anti-mouse IgG1 (Alexa FluorTM 488) (Thermo Fisher Scientific, catalog number: A-21121)
b. Goat anti-Mouse IgM heavy chain (Alexa FluorTM 546) (Thermo Fisher Scientific, catalog number: A-21045)
c. Goat anti-Mouse IgG2b (Alexa FluorTM 647) (Thermo Fisher Scientific, catalog number: A-21242)
d. Goat anti-Rabbit IgG (H+L) (Alexa FluorTM 750) (Thermo Fisher Scientific, catalog number: A-21039)
Reagents
1. 2-Methylbutane (Thermo Fisher Scientific, catalog number: 126470250)
2. Liquid nitrogen (Linde Gas & Equipment Inc, catalog number: NI 4.8LC23022SW)
3. Triton X-100 (MilliporeSigma, catalog number: T8787)
4. Normal goat serum (Thermo Fisher Scientific, catalog number: PCN5000)
5. Bovine serum albumin (BSA) (Equitech-Bio, catalog number: BAC62)
6. SlowFade Diamond antifade mountant (Thermo Fisher Scientific, catalog number: S36963)
7. Fisher Healthcare Tissue-PlusTM O.C.T. compound (Fisher Scientific, catalog number: 23-730-571)
8. Sodium chloride (NaCl) (MilliporeSigma, catalog number: S9625)
9. Potassium chloride (KCl) (MilliporeSigma, catalog number: P4504)
10. Sodium phosphate dibasic (Na2HPO4) (MilliporeSigma, catalog number: S0876)
11. Potassium phosphate monobasic (KH2PO4) (MilliporeSigma, catalog number: P5379)
12. 4',6-Diamidino-2-phenylindole dihydrochloride, 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) (Millipore Sigma, catalog number: D9542)
13. Dimethylformamide (MilliporeSigma, catalog number: 227056)
Solutions
1. Phosphate buffered saline (PBS) (see Recipes)
2. PBS + 0.1% Triton X-100 (see Recipes)
3. 0.5% BSA in 1× PBS (see Recipes)
4. Blocking solution (see Recipes)
5. Primary antibody solution (see Recipes)
6. Secondary antibody solution (see Recipes)
7. DAPI stock solution (see Recipes)
8. DAPI working solution (see Recipes)
Recipes
Note: Be precise about the ingredients (e.g., buffer or media), quantities, and conditions established for your experiments. Note that the omission of minor details from recipes (e.g., type of water used or storage conditions) might lead to the failure of the experiment.
1. Phosphate buffered saline (PBS) 1×
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 137 mM | 8 g |
| KCl | 2.7 mM | 0.2 g |
| Na2HPO4 | 10 mM | 1.44 g |
| KH2PO4 | 1.8 mM | 0.245 g |
| Deionized water | n/a | to 1,000 mL |
2. PBS + 0.1% Triton X-100
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS 1× (Recipe 1) | 1× | 4.995 mL |
| Triton X-100 | 0.1% | 5 μL |
| Total | 5 mL |
3. 0.5% BSA in 1× PBS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 0.5% | 250 mg |
| PBS 1× (Recipe 1) | 1× | to 50 mL |
Store at 4 °C for the duration of your experiment.
4. Blocking solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.5% BSA in 1× PBS (Recipe 3) | 1× | 315 μL |
| Normal goat serum | 10% | 35 μL |
Scale up when preparing multiple slides.
5. Primary antibody solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.5% BSA in 1× PBS (Recipe 3) | 1× | ~315 μL (per slide) |
| Normal goat serum | 2% | 7 μL |
| Primary antibody (four antibodies total) | 1:50 | 7 μL (per antibody per slide) |
Scale up when preparing multiple slides.
6. Secondary antibody solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.5% BSA in 1× PBS (Recipe 3) | 1× | ~315 μL (per slide) |
| Normal goat serum | 2% | 7 μL |
| Secondary antibody (four antibodies total) | 1:500 | 0.6 μL (per antibody per slide) |
Scale up when preparing multiple slides.
7. DAPI stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DAPI | 14.3 mM | 5 mg |
| Dimethylformamide | 12.87 M | 1 mL |
Mix to dissolve. It may take some time to completely dissolve. Protect from light, aliquot, and store at -20 °C.
8. DAPI working solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DAPI stock solution | 600 nM | 4 μL |
| PBS | 1× | 100 mL |
Store at 4 °C in a brown bottle or a bottle covered in foil. This should be stable for at least 6 months.
Laboratory supplies
1. VWR® Premium Superfrost® Plus microscope slides (VWR, catalog number: 48311-703)
2. VWR VistaVisionTM cover glasses, no. 1½ (VWR, catalog number: 16004-322)
3. ImmEdge® hydrophobic marker (Vector Laboratories, catalog number: NC9545623)
4. Coplin jar (Fisher Scientific, catalog number: E94)
5. Empty pipette tip box (Thermo Fisher Scientific, catalog number: 2069-05 or similar)
6. Paper towels (Kimberly-Clark ProfessionalTM, catalog number: 01804 or similar)
7. Dumont #7 curved forceps (Roboz, catalog number: RS-5047 or similar)
8. McPherson-Vannas curved spring micro scissors (Roboz, catalog number: RS-5631 or similar)
9. Cell lifters (Alkali Scientific, catalog number: TC7023 or similar)
10. Nail polish (Sally Hansen, catalog number: SALLYH2 or similar; avoid any glitter or metallic pigment colors)
Equipment
1. Leica CM1950 cryostat (Leica Biosystems, model: CM1950)
2. Confocal microscope (Leica Biosystems, model: Stellaris 5)
3. Wacom Intuos small Bluetooth tablet (Wacom, model: CTL4100WLK0)
Software and datasets
1. LAS X (Leica Biosystems)
2. FLIKA (version 0.3.1, free to use, available at https://flika-org.github.io/)
3. QuantiMus (free to use, available at https://quantimus.github.io/)
4. Image J (National Institutes of Health, version 1.54k, 15/10/2024)
Procedure
文章信息
稿件历史记录
提交日期: Dec 17, 2024
接收日期: Mar 3, 2025
在线发布日期: Mar 20, 2025
出版日期: Apr 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Matias, C., Yamada, C., Movila, A. and Brault, J. J. (2025). Optimizing Confocal Imaging Protocols for Muscle Fiber Typing in the Mouse Masseter Muscle. Bio-protocol 15(7): e5267. DOI: 10.21769/BioProtoc.5267.
分类
细胞生物学 > 细胞成像 > 共聚焦显微镜
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










