- EN - English
- CN - 中文
Baculovirus and Plasmid Vector-Mediated Gene Transfer in Daphnia magna Cells in Primary Embryonic In Vitro Cultures in an Optimized Microenvironment: Methods and Protocols
在优化微环境中利用杆状病毒和质粒载体介导的大型蚤胚胎原代细胞基因转导:方法与方案
发布: 2025年04月05日第15卷第7期 DOI: 10.21769/BioProtoc.5266 浏览次数: 1759
评审: Alberto RissoneSrestha DasguptaKeisuke Tabata

相关实验方案

利用睡美人转座子系统在RAW264.7巨噬细胞中构建可四环素诱导的基因表达模型以研究吞噬作用
Parsa Kamali and Gregory D. Fairn
2025年02月05日 2016 阅读
Abstract
Daphnia magna is a well-established model organism in ecotoxicology, environmental monitoring, and genetics due to its sensitivity to pollutants, its pivotal role in freshwater ecosystems, and its well-characterized genome. Despite its extensive use in these fields, there is a notable lack of established protocols for developing primary cell culture systems and conducting transgenic studies in Daphnia spp. This study addresses these gaps by optimizing a medium and standardizing protocols for primary cell culture and transgenic experiments in D. magna. Primary cell cultures were established from both D. magna embryos and whole organisms, with medium optimization verified using XTT assay. Cell viability was sustained for over two months using a modified Schneider’s insect medium enriched with FBS, glucose, MEM vitamin mix, and selenium. DNA replication and cell proliferation were confirmed through BrdU labeling. Both mechanical and enzymatic passaging methods were compared, resulting in 20% and 10% cell attachment, respectively. For transgenic applications, this study successfully standardized plasmid-mediated lipofection and baculovirus-mediated transduction, achieving success rates of 52% and 45%. These findings represent a pioneering effort in D. magna embryonic cell culture, offering a reliable in vitro platform for future biological research, including ecotoxicological and epigenetic investigations. The established protocols and optimized cell culture medium have significant implications for advancing crustacean cell line research and transgenic model development, enhancing our understanding of biological processes in controlled laboratory environments.
Key features
• Optimized primary embryonic cell culture system for Daphnia magna ensures extended viability and minimized contamination compared to adult-derived cultures.
• Modified Schneider’s insect medium (MSIM) with precise supplement concentrations enhances Daphnia cell viability, supporting applications in toxicity testing and genetic modifications.
• Efficient transfection and baculovirus-based transduction protocols enable robust transgene expression, validated through fluorescence microscopy and promoter-specific activity.
• Comparative subculture techniques demonstrate improved reattachment rates with gentle pipetting, providing insights into non-enzymatic handling for fragile aquatic cells.
Keywords: Daphnia magna (大型蚤)Graphical overview
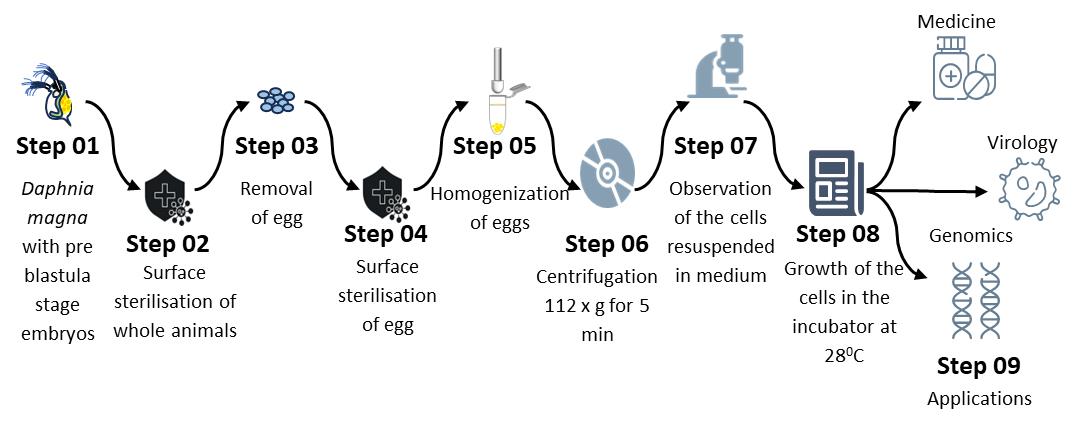
Primary cell culture development from Daphnia magna embryos
Background
Crustaceans, encompassing ~67,000 species, include Daphnia, a keystone species in freshwater ecosystems known for their adaptability, parthenogenetic reproduction, and utility in evolutionary and ecotoxicological studies. Despite Daphnia’s potential as a model organism, the lack of established cell lines limits in vitro research and pathogen studies [1]. Challenges in crustacean cell culture primarily stem from the absence of suitable media [2,3], which has prompted modifications of commercially available media to optimize cell viability and microenvironments.
Previous attempts to develop crustacean cell lines have faced poor cell survival [1–3]. Notable successes include hybrid lines like PmLyO-Sf9 [4], but efforts for Daphnia magna have yielded limited results [5,6], emphasizing the need for optimized protocols. Advances in Daphnia genomic tools and transgenic methods, including RNAi [7], targeted mutagenesis [8], and genome editing [9], have paved the way for genetic manipulation. Cellfectin has shown higher efficiency than lipofectamine in Arthropoda [10]. The EF1α-DsRed plasmid, modified as pRCS21-EF1α1(UTR), achieved 0.67% transgenic D. magna via microinjection [11]. Baculovirus expression systems, mainly Autographa californica MNPV, enhance protein expression using polyhedrin or p10 promoters. However, challenges persist in transfection and transduction due to low cell numbers and attachment efficiency in novel cultures.
This study establishes a standardized protocol for developing primary D. magna embryonic cell cultures using a modified medium to enhance cell survival. Transfection and transduction protocols are optimized to create an immortalized D. magna cell line, offering broad applications in crustacean research and biotechnology.
Materials and reagents
Biological materials
1. Daphnia magna: Daphnia Research Facility (DRF), National Centre for Aquatic Animal Health (NCAAH; https://www.ncaah.ac.in), Cochin University of Science and Technology (CUSAT; https://cusat.ac.in/), Kerala, India
2. pCS-EF1α1-DSRed2 vector, obtained from Dr. Hajime Watanabe, Osaka University, Japan [10]
3. Recombinant baculovirus expression system (BacIe1-GFP and BacP2-GFP), developed in NCAAH, CUSAT [2,3]
4. SF9 cells, maintained at NCAAH, CUSAT
Reagents
1. MEM vitamins (MEM) (Gibco, catalog number: 11120052)
2. 1× PBS (Gibco, catalog number: 10010023)
3. Schneider’s insect medium (Gibco, catalog number: 21720024)
4. Fetal bovine serum (FBS) (Gibco, catalog number: A5670701)
5. 1× Leibovitz’s L-15 medium (L-15) (Sigma-Aldrich, catalog number: L1518)
6. TNM-FH insect medium (TNMFH) (Sigma-Aldrich, catalog number: T1032)
7. Grace’s insect medium (GIM) (Sigma-Aldrich, catalog number: G9771)
8. Minimal Essential Medium (MEM) (Sigma-Aldrich, catalog number: 56416C)
9. Dulbecco’s modified Eagle medium (DMEM) (Sigma-Aldrich, catalog number: D6546)
10. Roswell Park Memorial Institute Medium (RPMI) (Sigma-Aldrich, catalog number: R8758)
11. XTT (Invitrogen, catalog number: X6493)
12. Glucose, ≥99.5% (Sigma-Aldrich, catalog number: G7021)
13. Trehalose, ≥99% (Sigma-Aldrich, catalog number: T0167)
14. Sucrose, ≥99% (Sigma-Aldrich, catalog number: S5390)
15. Amino acids mix (MEM) (Gibco, catalog number: 11130036)
16. Cholesterol (Sigma-Aldrich, catalog number: C4951)
17. Sodium selenite, ≥98% (Sigma-Aldrich, catalog number: S5261)
18. Trypsin-EDTA solution (Sigma-Aldrich, catalog number: T4049)
19. Acridine orange (AO) (Invitrogen, catalog number: A1301)
20. Ethidium bromide (EB) (Invitrogen, catalog number: 15585011)
21. BrdU (5-Bromo-2’-deoxyuridine) (TCI, catalog number: B1575)
22. Mouse monoclonal anti-BrdU antibody (Sigma-Aldrich, catalog number: SAB4700630)
23. Alexa Fluor 488 (Jackson ImmunoResearch Labs, catalog number: 115-545-003)
24. DAPI (Thermo Fisher Scientific, catalog number: D3571)
25. Cellfectin (CellfectinTM II) (Invitrogen, catalog number: 10362100)
26. Selenium dioxide (SeO2) (Sigma-Aldrich, catalog number: 204315)
27. Penicillin (100 IU/mL) and streptomycin (100 μg/mL) solution (Roche, Merk, catalog number: 11074440001)
Solutions
1. ADaM media (see Recipes)
2. 0.05% sodium hypochlorite (see Recipes)
3. 70% ethanol (see Recipes)
4. 2 M HCl (see Recipes)
5. 0.1 M sodium borate (see Recipes)
6. 0.2% Triton X-100 (see Recipes)
7. 3% BSA (see Recipes)
8. 0.05% trypsin-EDTA (see Recipes)
Recipes
1. ADaM media
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CaCl2·2H2O | 0.2705 g/L | 23 mL of Stock A (117.6 g/L CaCl2·2H2O) |
| NaHCO3 | 0.0554 g/L | 22 mL of Stock B (25.2 g/L NaHCO3) |
| SeO2 | 7 μg/L | 1 mL of Stock C (0.07 g/L SeO2) |
| Distilled water | Up to 10 L |
2. 0.05% sodium hypochlorite
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium hypochlorite powder | 0.05% (w/v) | 0.05 g (for 10 mL) |
| Distilled water | n/a | Up to 10 mL |
| Total | n/a | 10 mL |
3. 70% ethanol
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol (100%) | 70% (v/v) | 7 mL |
| Distilled water | n/a | 3 mL |
| Total | n/a | 10 mL |
4. 2 M HCl
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| HCl (12 M) | 2 M | 1.67 mL |
| Distilled water | n/a | 8.33 mL |
| Total | n/a | 10 mL |
5. Sodium borate (0.1 M)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sodium borate | 0.1 M | 0.382 g |
| Distilled water | n/a | 9.618 mL |
| Total | n/a | 10 mL |
6. 0.2% Triton X-100
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Triton X-100 | 0.2% | 20 mg |
| Distilled water | n/a | 10 mL |
| Total | n/a | 10 mL |
7. 3% BSA
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 3% BSA | 3% | 0.3 g |
| Distilled water | n/a | 10 mL |
| Total | n/a | 10 mL |
8. 0.05% trypsin-EDTA
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Trypsin-EDTA stock | 0.25% | 2 mL |
| Distilled water | n/a | 8 mL |
| Total | n/a | 10 mL |
Laboratory supplies
1. 1.5 mL microcentrifuge tubes (MCTs) (Tarson, catalog number: 500010)
2. 2 mL microcentrifuge tubes (MCTs) (Tarson, catalog number: 500020)
3. Needles (Sigma-Aldrich, catalog number: Z117013)
4. Glass homogenizer (Sigma-Aldrich, catalog number: D8938)
5. 96-well plate (TPP, catalog number: Z707902)
6. 24-well plate (TPP, catalog number: Z707791)
7. Coverslips (Sigma-Aldrich, catalog number: Z355445)
8. Pipette tips: 1–10, 10–100, 100–1,000 μL (Sigma-Aldrich, catalog numbers: Z678295, Z678325, AXYT1000CR)
Equipment
1. Fluorescent microscope (Invitrogen, model: AMF7000, EVOSTM M7000 Imaging System)
2. Centrifuge (Eppendorf, model: 5424R, catalog number: 5404000138)
3. Dark incubator, 28 °C (Thermo Fisher Scientific, catalog number: 3120, model: Forma Series II)
4. Laminar airflow (Thermo Scientific, catalog number: 51025413, MSC-AdvantageTM Class II Biological Safety Cabinets)
Software and datasets
1. SPSS® software (version 21; SPSS Inc., Chicago, IL, USA)
Procedure
文章信息
稿件历史记录
提交日期: Dec 25, 2024
接收日期: Mar 2, 2025
在线发布日期: Mar 24, 2025
出版日期: Apr 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Sreevidya, C. P., Balakrishnan, S. and Puthumana, J. (2025). Baculovirus and Plasmid Vector-Mediated Gene Transfer in Daphnia magna Cells in Primary Embryonic In Vitro Cultures in an Optimized Microenvironment: Methods and Protocols. Bio-protocol 15(7): e5266. DOI: 10.21769/BioProtoc.5266.
分类
环境生物学 > 寄生生物
细胞生物学 > 模式生物培养 > 胚胎筛选
细胞生物学 > 基于细胞的分析方法 > 基因表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











