- EN - English
- CN - 中文
A Human Cervix Chip for Preclinical Studies of Female Reproductive Biology
用于女性生殖生物学前沿研究的人源子宫颈芯片模型
发布: 2025年04月05日第15卷第7期 DOI: 10.21769/BioProtoc.5262 浏览次数: 2064
评审: Alka MehraHsih-Yin TanIbrahim AlabriAnonymous reviewer(s)
Abstract
Pathological conditions of the cervix ranging from cervical cancer to structural dysfunction associated with preterm labor all have limited treatment options. Thus, there is a need for physiologically relevant preclinical models that recapitulate the structure and function of this human organ. Here, we describe a protocol for engineering and studying a highly functional in vitro model of the human cervix that is composed of a commercially available, dual-channel, microfluidic, organ-on-a-chip (Organ Chip) device lined by primary cervical epithelial (CE) cells interfaced across a porous membrane with cervical stromal cells. The provision of dynamic and customized media flow through both the epithelial and stromal compartments results in cell growth and differentiation, including the accumulation of a thick mucus layer overlying the epithelium. The resulting model closely mimics the structure, epithelial barrier, mucus composition and structure, and biochemical properties of the in vivo human cervix, as well as its responsiveness to female hormones, pH, and microbiome. This Cervix Chip protocol also includes noninvasive techniques for longitudinal monitoring of the live 3D tissue model. The Cervix Chip offers a powerful preclinical platform for replicating in vivo cervical physiology, studying disease mechanisms, and facilitating the development of new therapeutics and diagnostics.
Key features
• Creates a functional and physiologically responsive 3D tissue model of the human cervix including a living epithelial–stromal interface.
• Enables longitudinal and endpoint analysis of the epithelial and stromal environment and their respective secretions independently.
• Allows extended clinically relevant studies, such as assessment of tissue barrier function and mucus production as well as co-culture with microbiome and pathogens.
• Uses a commercially available dual-channel microfluidic chip and automated culture system (ZoëTM Culture Module, Emulate Inc., USA).
Keywords: Organ-on-a-Chip (器官芯片)Graphical overview
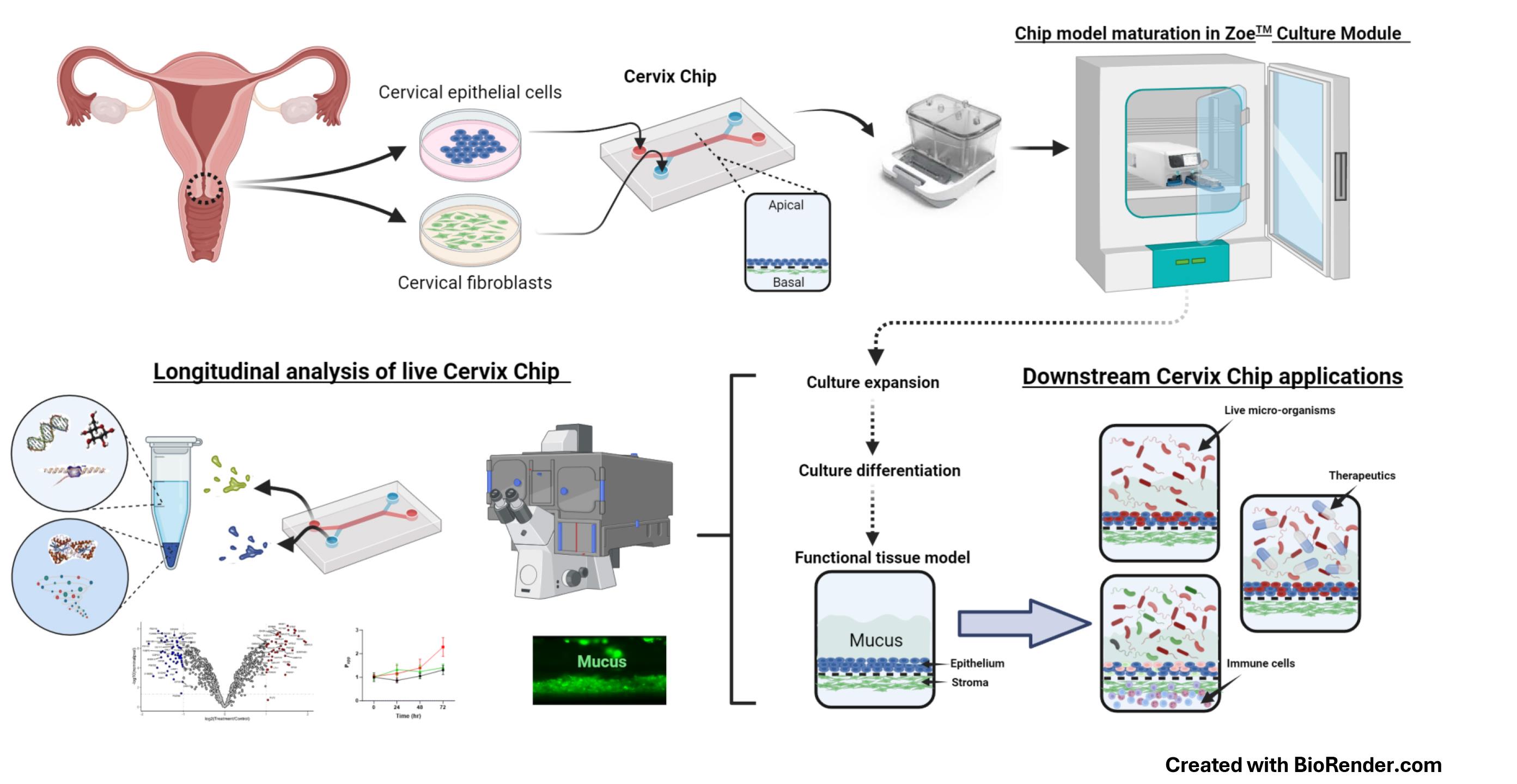
Human Cervix Chip design, culture, analysis, and applications
Background
The human cervix is a specialized mucosal organ that provides protection against external pathogens while supporting fertilization and fetal development in the female reproductive tract. Yet, little is known about its physiology and pathophysiology. In fact, progress in the broader area of female reproductive and sexual health has lagged behind other fields, and this is largely due to persistent knowledge gaps and a lack of concerted efforts in both basic and translational research. The scarcity of new, more effective therapeutics for female diseases affects approximately one-third of women globally, placing a significant economic burden on healthcare systems [1]. Studying the physiology and pathophysiology of the female reproductive tract ex vivo has been challenging due to the complexity of these organs and their microenvironment, as well as the limitations of conventional animal studies, tissue explants, and standard cell cultures. While these models are convenient, widely used, and standardized, they fail to faithfully replicate the structure, function, and physiological responses of the human female organs due to species differences or overly simplified configurations [2–5].
Microfluidic Organ-on-a-Chip (Organ Chip) technology provides a promising platform for developing in vitro models that mimic human physiology and disease states at the cellular, tissue, organ, and even organism levels [6–9]. We recently described the development of an Organ Chip model of the human cervix (Cervix Chip) [6] using a commercially available dual-channel microfluidic chip (Chip-S1, Emulate Inc., USA) and their ZoëTM culture module, which operates automatically inside a standard tissue culture incubator. The Chip-S1 is composed of two parallel microfluidic channels, each less than 1 mm wide, separated by an extracellular matrix (ECM)-coated microporous membrane. The Cervix Chip is created by lining one side of this membrane with human primary cervical epithelial (CE) cells, while primary human cervical stromal cells are cultured on the opposite side of the same membrane in the parallel channel (see Graphical overview). Both cell types are then expanded and differentiated under dynamic flow conditions to form a functional cervical epithelium and underlying stroma that closely resembles the epithelial–stromal interface of the cervical mucosa in vivo. The human Cervix Chip exhibits key features of the living cervix, including the formation of a functional tissue barrier, the accumulation of a thick mucus layer overlying the epithelium, and responsiveness to female hormones, environmental factors, and both healthy and dysbiotic microbiome. Additionally, the Cervix Chip protocol allows for continuous analysis of molecular, cellular, and functional properties, as well as endpoint analyses on fixed tissues. The transparent chip design enables noninvasive optical imaging, and the ability to control flow dynamics allows for stable co-cultures for days with live microorganisms (e.g., bacteria) as well as the collection of effluents from the epithelial and stromal channels individually for off-chip analysis (e.g., similar to sampling cervical discharge and blood or interstitial fluid, respectively).
Previous protocols for engineering 3D cervical tissues in vitro did not offer such versatility or the ability to conduct longitudinal studies without interrupting cultures [10]. Performing complex co-culture studies with multiple cell types or microorganisms has also been challenging in static culture models [11,12]. The Cervix Chip overcomes these limitations and provides a robust platform for investigating tissue dynamics and host–microbiome interactions. Importantly, because the Organ Chip devices and automated chip culture instruments we used are commercially available, this approach can be pursued in any lab able to acquire this equipment. The availability of mass-manufactured chips and control systems also enables high reproducibility and standardization, making it valuable for academic, clinical, and pharmaceutical applications.
The human Cervix Chip protocol described here has been previously validated in a study that successfully modeled bacterial vaginosis (BV) in vitro. BV is a chronic condition affecting over 25% of women, characterized by a shift in the vaginal microbiome from Lactobacillus crispatus dominance to a diverse community of anaerobic bacteria, including Gardnerella vaginalis and Atopobium vaginae [13–15]. This study showed that the Cervix Chip is a useful tool for studying complex disease conditions of the cervical mucosa, such as BV. We have also pursued a similar approach to create an Organ Chip of the human vagina [7]. This can be extended to create models of other organs of the female urogenital and reproductive tract (e.g., uterus, fallopian tube, ovary, urethra, and bladder).
Materials and reagents
Biological materials
1. Human cervical epithelial cells (LifeLine Cell Technology, catalog number: FC-0080)
2. Human cervical stromal cells, de-identified donor human hysterectomy or cadaver tissue obtained following approval by the Institutional Review Board of Wyss Institute for Biologically Inspired Engineering at Harvard University (Protocol number: IRB22632) and Mass General Brigham (Protocol number: 2015P001859)
Reagents
1. Trypsin 0.05%-EDTA 1× (Gibco, catalog number: 25300-054)
2. Defined trypsin inhibitor (DTI) (Thermo Fisher, catalog number: R007100)
3. Cervical cell culture basal medium (Lifeline Cell Technology, catalog number: LM-0055)
4. Cervical Cell Culture Growth kit; includes HLL supplement LifeFactor, HAS, linoleic acid, lecithin, rh insulin LifeFactor, rh EGF LifeFactor, L-glutamine LifeFactor, epinephrine LifeFactor, extract PTM LifeFactor, hydrocortisone hemisuccinate LifeFactor, triiodothyronine LifeFactor, and PS transferrin LifeFactor reagents (LifeLine Cell Technology, catalog number: LL-0072)
5. Fibroblast basal medium (ATCC, catalog number: PCS-201-030)
6. Fibroblast Growth kit, low serum; includes L-glutamine rh FGF basic, rh insulin, hydrocortisone, ascorbic acid, and FBS reagents (ATCC, catalog number: PCS-201-041)
7. Penicillin-streptomycin (Gibco, catalog number: 15070063)
8. Primocin (InvivoGen, catalog number: ant-pm-2)
9. Ascorbic acid (ATCC, catalog number: PCS-201-040)
10. Beta-estradiol (Sigma, catalog number: E8875)
11. Collagen I bovine (Advanced BioMatrix, catalog number: 5005-100ML)
12. Collagen IV, human placenta Col IV powder (Sigma-Aldrich, catalog number: C7521-10MG)
13. Fibronectin, human (Corning, catalog number: 356008)
14. Dispase (Worthington Biochemical Corporation, catalog number: LS02109)
15. Collagenase V (Sigma, catalog number: C-9263)
16. Acetic acid (Sigma-Aldrich, catalog number: A6283)
17. RPMI 1640 medium (Thermo Fisher Scientific, catalog number: 21875-034)
18. Advanced DMEM/F12 (Thermo Fisher Scientific, catalog number: 12634010)
19. Fetal bovine serum (FBS) (Thermo Fisher, catalog number: A3840001)
20. HBSS, pH 7.4 (Thermo Fisher, catalog number: 14025076)
21. DPBS (Thermo Fisher Scientific, catalog number: 14190144)
22. Calcium chloride (CaCl2) (anhyd.) (Thermo Fisher, catalog number: 423525000)
23. Magnesium chloride (MgCl2·6H2O) (Thermo Fisher, catalog number: 413415000)
24. Magnesium sulfate (MgSO4·7H2O) (Thermo Fisher, catalog number: 423905000)
25. Potassium chloride (KCl) (Thermo Fisher, catalog number: 424090250)
26. Potassium phosphate monobasic (KH2PO4) (Thermo Fisher, catalog number: 424205000)
27. Sodium chloride (NaCl) (Thermo Fisher, catalog number: 424295000)
28. ER-1TM (EmulateTM Inc., USA, catalog number: ER105)
29. ER-2TM (EmulateTM Inc., USA, catalog number: ER225)
30. Trypan blue solution (Sigma-Aldrich, catalog number: T8154-200ML)
31. Collagenase IV (Gibco, catalog number: 17104019)
32. TrypLE (Thermo Fisher, catalog number: 12605010)
33. N-acetylcysteine (NAC) (Sigma, catalog number: A9165)
34. 16% Paraformaldehyde (PFA) (Thermo Fisher Scientific, catalog number: 28908)
35. Cascade Blue® hydrazide, trilithium salt (Thermo Fisher, catalog number: C3239)
36. Fluorescent wheat germ agglutinin (WGA) (Invitrogen, catalog number: W11261)
37. Cell lysis buffer (QIAGEN, catalog number: 74034)
Solutions
1. Col IV stock solution (see Recipes)
2. Fibronectin stock solution (see Recipes)
3. Beta-estradiol stock solution (see Recipes)
4. 4% PFA solution (see Recipes)
5. HBSS [LB/-G] (see Recipes)
6. Cervical growth medium (see Recipes)
7. Fibroblast growth medium (see Recipes)
8. Cervical epithelium differentiation medium (see Recipes)
Recipes
Note: Prepare the growth media fresh every three days. Sterilize the HBSS [LB/-G] and growth media using the vacuum filtration system inside the biosafety cabinet before storage at 4 °C.
1. Col IV stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Collagen IV powder | 500 μg/mL | 10 mg |
| dH2O | n/a | 19.96 mL |
| Acetic acid | n/a | 40 μL |
| Total | n/a | 20 mL |
Filter sterilize the solution using a 0.2 μm filter. Store at -20 °C in small-volume aliquots to avoid freeze-thaw cycles.
2. Fibronectin stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Fibronectin | 100 μg/mL | 1 mg |
| DPBS | n/a | 10 mL |
| Total | n/a | 10 mL |
Make the solution under aseptic conditions. Store aliquots at -80 °C.
3. Beta-estradiol stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Beta-estradiol | 1 μg/mL | 1 mg |
| 100% ethanol | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
Store at -80 °C.
4. 4% PFA solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 16% PFA | 4% | 1 mL |
| DPBS | n/a | 3 mL |
| Total | n/a | 4 mL |
Make fresh solution before tissue fixation.
5. HBSS [LB/-G]
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CaCl2 | 140 mg/L | 140 mg |
| MgCl2·6H2O | 100 mg/L | 100 mg |
| MgSO4·7H2O | 100 mg/L | 100 mg |
| KCl | 400 mg/L | 400 mg |
| KH2PO4 | 60 mg/L | 60 mg |
| NaCl | 8 g/L | 8 g |
| H2O | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
6. Cervical growth medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Cervical cell culture basal medium | n/a | 474 mL |
| Cervical Cell Culture Growth kit | ||
| HLL supplement LifeFactor | 1.25 mL | |
| HSA | 500 μg/mL | |
| Linoleic acid | 0.6 μM | |
| Lecithin | 0.6 μg/mL | |
| rh Insulin LifeFactor | 5 μg/mL | 0.5 mL |
| rh EGF LifeFactor | 6 mM | 0.5 mL |
| L-glutamine LifeFactor | 1 μM | 15 mL |
| Epinephrine LifeFactor | 0.4% | 0.5 mL |
| Extract PTM LifeFactor | 100 ng/mL | 2 mL |
| Hydrocortisone hemisuccinate LifeFactor | 10 nM | 0.5 mL |
| Triiodothyronine LifeFactor | 5 μg/mL | 0.5 mL |
| PS Transferrin LifeFactor | 0.5 mL | |
| Penicillin-streptomycin | 50 U/mL | 5 mL |
| Total | n/a | 500 mL |
7. Fibroblast growth medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Fibroblast basal medium | n/a | 464 mL |
| Fibroblast Growth kit, low serum | ||
| L-glutamine | 7.5 mM | 18.75 mL |
| rh FGF basic | 5 ng/mL | 0.5 mL |
| rh Insulin | 5 μg/mL | 0.5 mL |
| Hydrocortisone | 1 μg/mL | 0.5 mL |
| Ascorbic acid | 50 μg/mL | 0.5 mL |
| FBS | 2% | 10 mL |
| Penicillin-streptomycin | 50 U/mL | 5 mL |
| Total | n/a | 500 mL |
8. Cervical epithelium differentiation medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Cervical growth medium | n/a | 490 mL |
| Beta-estradiol stock solution (-80 °C) | 5 nM | 0.5 mL |
| Ascorbic acid (50 mg/mL) | 50 μg/mL | 0.5 mL |
| Total | n/a | 500 mL |
Laboratory supplies
1. Basic Research Chip kit, 24 PK containing Chip-S1 and Pod® devices (Emulate Inc. USA) (Figure 1a)
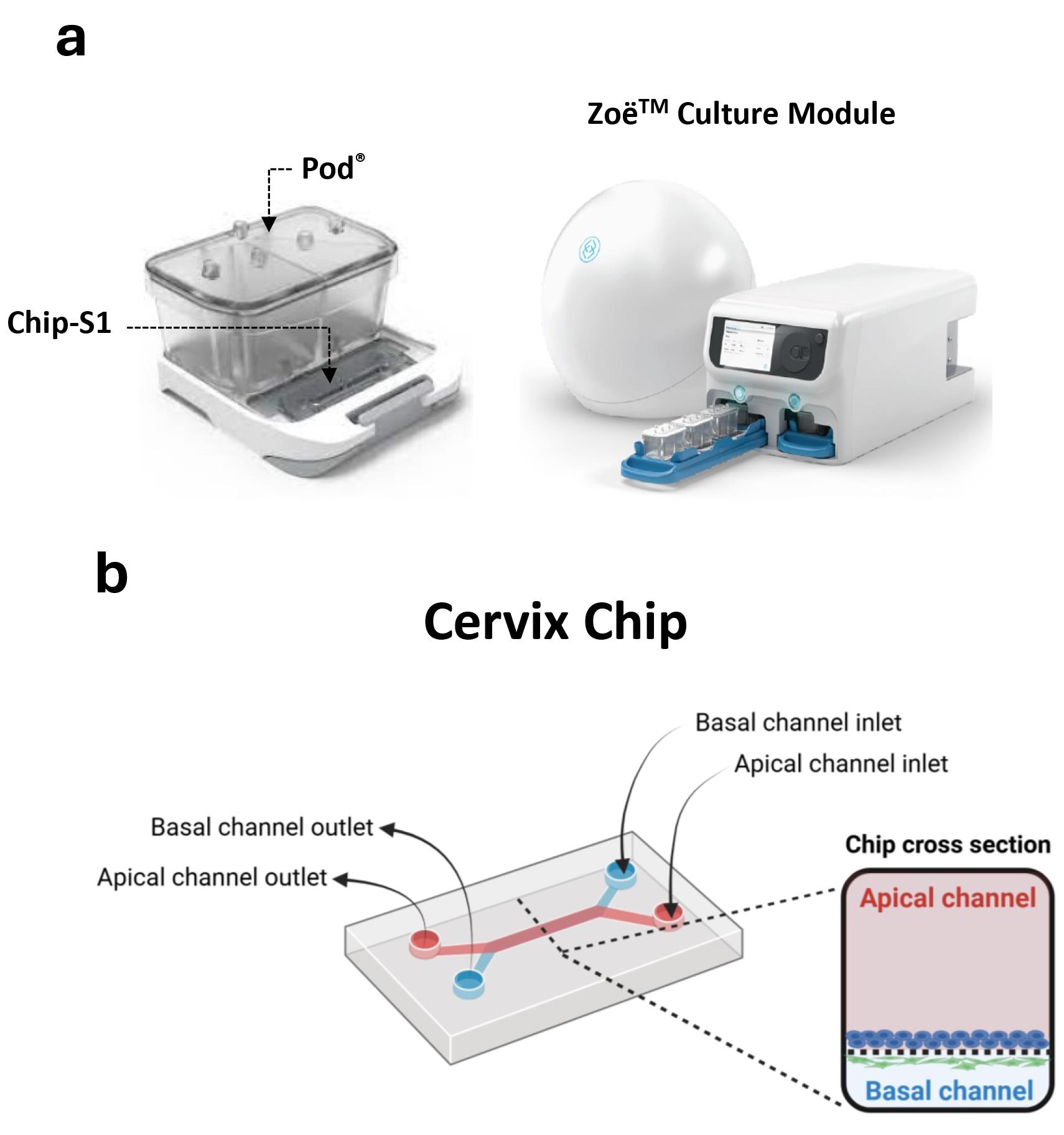
Figure 1. Commercial microfluidic chip device and automated culture module for creating the cervix chip. (a) Chip-S1 mounted on the microfluidic Pod® (left) for culture in ZoëTM culture module (right). (b) Schematic of the dual-channel cervix chip showing the inlets and outlets of the apical and basal channels as well as the cross-section view of the chip.
2. T75 flask (Thomas Scientific, catalog number: 21A00M452)
3. 35 mm cell culture dish (Corning, catalog number: 353001)
4. Conical centrifuge and microcentrifuge tubes
5. Hemocytometer
6. Steriflip vacuum filter units (Fisher Scientific, catalog number: SCGP00525)
7. Vacuum filtration system (Corning, catalog number: 431096)
8. Cell sieve (Corning, catalog number: 431752)
9. Square clear Petri dish (Thomas Scientific, catalog number: 688161)
10. 96-well assay block, 2 mL (Costar, catalog number: 04624000)
11. 96-well half area black flat bottom microplate (Corning, catalog number: 3881)
12. Disposable scalpel (VWR, catalog number: 76457-484)
13. Test tube rack (Thermo Fisher Scientific, catalog number: 5970-0120)
14. U-PLEX® Biomarker Assay kit (Mesoscale Discovery, catalog number: K15067L-1)
Equipment
1. Class II Biosafety cabinet (Thermo Fisher Scientific, model: 1300 Series A2)
2. Tissue culture incubator (VWR, model: Water Jacketed CO Incubator, catalog number: 10810-878)
3. ZoëTM culture module (Emulate Inc., model: CM-1) (Figure 1a)
4. Phase contrast/fluorescence microscope (Zeiss, model: Axio Observer Z1)
5. Condenser (Zeiss, model: 0.35 NA, 424241-0000-000)
6. Benchtop centrifuge (Eppendorf, model: 5804 R)
7. UV lamp (Nailstar, model: NS-01-US)
8. Multimode plate reader (BioTek NEO, model: Gen5 3.11)
9. MSD microplate reader (Mesoscale Discovery, model 1300 MESO QuickPlex SQ 120MM)
Software and datasets
1. Prism v8.1.2 (GraphPad Software Inc., San Diego, CA)
2. Fiji version v1.8 ( https://doi.org/10.1038/nmeth.2019)
3. Discovery Workbench v4.0.12
Procedure
文章信息
稿件历史记录
提交日期: Dec 18, 2024
接收日期: Mar 2, 2025
在线发布日期: Mar 18, 2025
出版日期: Apr 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Izadifar, Z. and Ingber, D. E. (2025). A Human Cervix Chip for Preclinical Studies of Female Reproductive Biology. Bio-protocol 15(7): e5262. DOI: 10.21769/BioProtoc.5262.
分类
生物工程 > 合成生物学
细胞生物学 > 组织分析 > 组织培养
细胞生物学 > 细胞分离和培养 > 微流体细胞培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link